- pubchem.ncbi.nlm.nih.gov - Fluorine
- britannica.com - Fluorine
- ncbi.nlm.nih.gov - The Fluoride Debate: The Pros and Cons of Fluoridation, Antoine Aoun, Farah Darwiche, Sibelle Al Hayek, Jacqueline Doumit
- ncbi.nlm.nih.gov - FLUORIDE: A REVIEW OF USE AND EFFECTS ON HEALTH, Domen Kanduti, Petra Sterbenk, Barbara Artnik
- lpi.oregonstate.edu - Fluoride
- sciencedirect.com - Fluoride, M. Abdollahi, F. Momen-Heravi
- sciencedirect.com - Fluorine, R. W. Kapp Jr.
- multimedia.efsa.europa.eu - Dietary Reference Values for the EU
- solen.cz - BONE FLUOROSIS, MUDr. Dagmar Opichalová, MUDr. Pavel Horák, CSc., MUDr. Věra Vavrdová, doc. MUDr. Martin Tichý, CSc.
Fluorine: what are its health effects? Symptoms of deficiency and excess + interesting facts

Fluoride is an important trace element in our bodies. It is a building block of bones and teeth. What other processes is it involved in? What role does it play in the prevention of tooth decay?
Article content
- Basic characteristics of fluorine
- What is the function of fluorine in the body?
- How does fluoride work in preventing tooth decay?
- Role of fluoride in the body
- What is the recommended daily intake of fluoride?
- Food and other sources of fluoride
- Fluoridation of water and food - what is the significance?
- Fluoride in toothpastes or as a dietary supplement
- How does fluoride deficiency and excess manifest itself?
- Fluorosis as a serious consequence of excessive fluoride
FLUOR: What should we know about it? What is its importance in the body?
Basic characteristics of fluorine
Fluorine is a non-metallic chemical element. It is not only abundant in nature, but is also one of the trace minerals present in the human body and important for maintaining health.
It has the chemical symbol F. This is derived from the Latin word fluorum.
Fluorine is named after the mineral fluorite, which is the main natural source of fluorine. The "fluo" part of the word means "to flow" in Latin and refers to the practical use of fluorite - it was added to metal ores to lower their melting point.
Fluorine is an element in Group 17 of the periodic table of chemical elements and is found in Period 2.
It belongs to the group of elements called halogens, which also includes chlorine, bromine, and iodine. The group was named for the ability of its elements to form salts (from the Greek hals - salt, gennaó - I form).
At standard pressure and temperature, fluorine is a pale yellow gas with an irritating smell. At lower temperatures, it turns into a yellow liquid.
Among the halogens, it is the lightest element and has the highest electronegativity. It is because of its high electronegativity that it is the most reactive element of all the elements in the periodic table.
It reacts with almost all elements (except argon, neon or helium) and also with most inorganic and organic substances.
Fluorine is also the strongest oxidizing agent. It reacts with many metals and coats them with a layer of fluoride.
Like other halogens, fluorine occurs as a diatomic molecule called F2.
A tabular summary of basic chemical and physical information about fluorine
| Name | Fluorine |
| Latin name | Fluorum |
| Chemical name | F |
| Classification of elements | Halogen |
| Grouping | Gas (at room temperature) |
| Proton number | 9 |
| Atomic mass | 18,998 |
| Oxidation number | -1 |
| Density | 1,696 g/l |
| Melting point | -219,67 °C (as F2) |
| Boiling point | -188,11 °C (as F2) |
Fluorine was first discovered in its compound hydrofluoric acid. However, the excessive reactivity of fluorine caused its discoverers considerable difficulty in isolating it from compounds to a pure element.
It was not until 1886 that the French chemist Henri Moissan succeeded in isolating elemental fluorine by low-temperature electrolysis.
Henri Moissan won the Nobel Prize in Chemistry for isolating elemental fluorine.
Fluorine is a relatively widespread element. It occurs naturally in the atmosphere, soil, water, rocks of volcanic origin and also in plants and animals.
It is the thirteenth most abundant element on Earth. It makes up 0.06-0.09% of the weight of the Earth's crust.
The highest concentrations of fluorine are found in areas rich in fluoride minerals, in volcanic areas, in industrial areas where fluorine compounds are released into the environment (coal combustion, ore processing) or even in places where fertilisers are produced and used.
Fluorine is found in all natural waters, including seawater. Its content in seawater is several times higher than in fresh water.
It occurs naturally only in the form of compounds. It is bound in molecules as the inorganic fluoride F-. It does not occur in free form because of its high reactivity.
Fluorine-containing minerals include the aforementioned fluorite (CaF2), as well as cryolite (Na3AlF6), fluorapatite (Ca5(PO4)3F), topaz, lepidolite and mica.
Elemental fluorine or its compounds are currently used in many fields.
For example:
- As an auxiliary agent to lower the melting point or viscosity in metal processing (aluminium or iron).
- To clean metals, polish or etch glass
- To produce Teflon or uranium fluoride (used in the nuclear power industry)
- As a refrigerant in refrigerators, air conditioners or fire extinguishers (use for this purpose is already restricted due to their contribution to ozone depletion)
- As a drinking water additive - water fluoridation
- As an additive in toothpastes
- For the manufacture of certain medicines

What is the function of fluorine in the body?
Fluorine is an important trace element for the human body. Although it is present in relatively small amounts in the body, it is essential for the proper functioning of several physiological processes.
In the body, fluorine occurs only in the form of an ion. It is the inorganic fluoride anion F-. Therefore, fluorine compounds are called fluorides.
The most important biological function of fluoride is the maintenance of healthy teeth and bones.
It accumulates in the hard tissues of the body, i.e. in the bones and teeth. There, together with calcium and phosphorus, it forms crystals of the minerals fluorapatite or fluorohydroxyapatite.
We are talking about the process of mineralisation which makes these tissues sufficiently strong and hard.
In this respect, fluorine performs the following functions:
- It is a key element in the development of teeth, as it helps their growth and formation.
- It acts as a preventive agent against tooth decay.
- It is used in the treatment of dental caries because it can slow or reverse the progression of existing carious lesions.
- It forms a protective layer on the surface of the teeth, thereby reducing the level of harmful effects of acids from food or acids produced by bacteria present in the oral cavity.
- It is important for maintaining the strength and firmness of teeth and tooth enamel.
- It helps to improve bone density and hardness, making bones stronger and more stable.
How does fluoride work in preventing tooth decay?
The effect of fluoride in maintaining the health and strength of teeth can be explained by three mechanisms:
- it promotes tooth mineralization
- prevents demineralisation of the teeth
- slows the growth of bacteria and reduces their effect
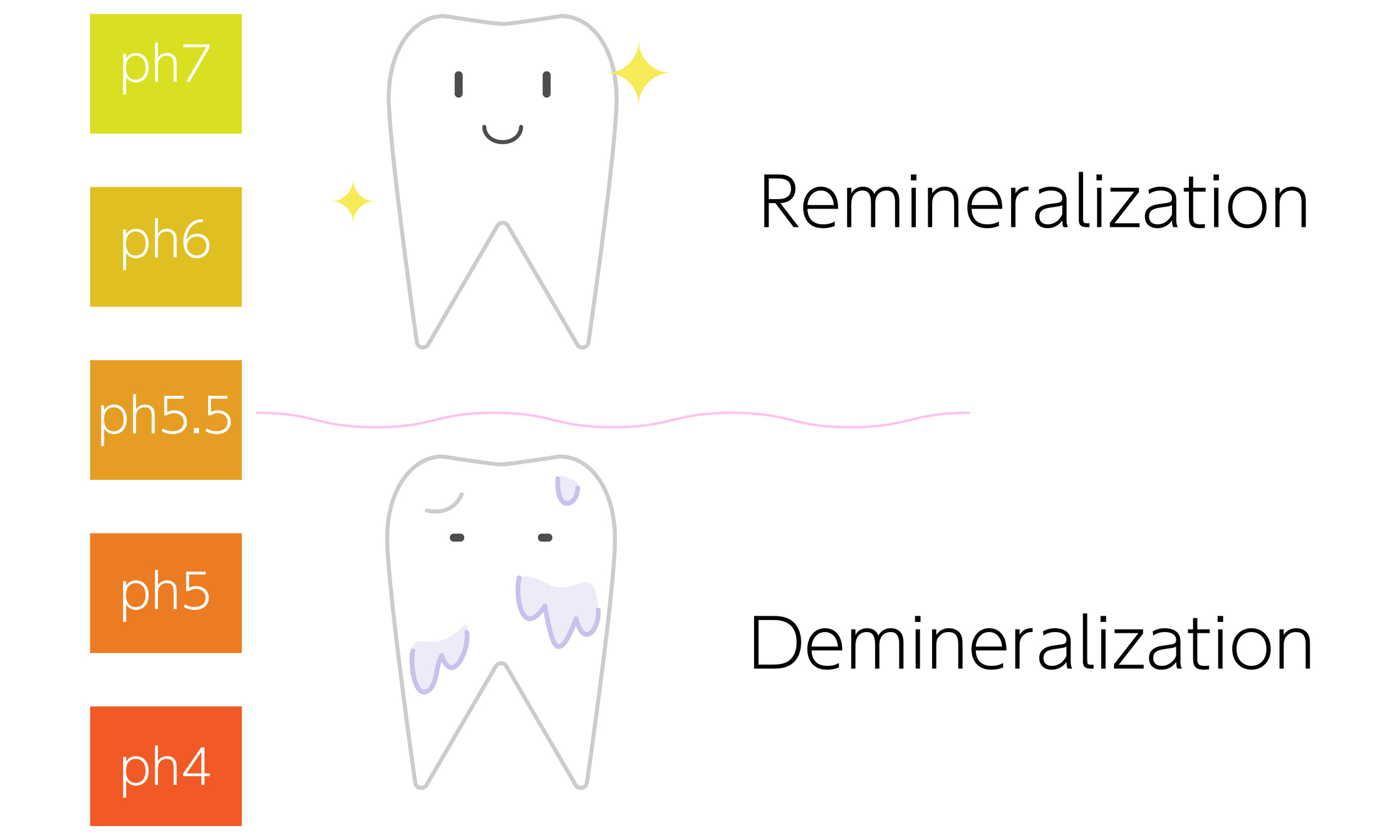
Teeth and tooth enamel are subject to the constantly recurring processes of demineralisation (the release of minerals from tooth tissues) and remineralisation (the re-deposition of minerals in tooth tissues) during growth and development, as well as during a person's lifetime.
Demineralisation causes a reduction in the strength and resistance of tooth enamel and can lead to tooth decay.
Bacteria present in the oral cavity play an important role in demineralisation. The bacteria metabolise sugar and produce lactic acid.
When the pH of saliva falls below the critical value of 5.5, the process of demineralisation begins and tooth decay can occur.
Demineralisation releases the mineral hydroxyapatite Ca10(PO4)6(OH)2 from the enamel. This is the most important building block of hard dental tissues and ensures their strength and hardness.
If fluoride is present in the oral cavity, it binds to the surface of the enamel crystals and protects them from dissolving. It can thus reduce the rate of mineral release, i.e. prevent demineralisation.
Subsequently, when the pH rises above a critical value, fluoride triggers the remineralisation process. It is absorbed into the enamel and contributes to the formation of the mineral fluorohydroxyapatite.
Remineralisation is a restorative process. It can only take place if sufficient quantities of the necessary substances are present in the saliva. One of these substances is fluoride.
The primary effect of fluoride is local. It is very important that it is present in saliva in sufficient concentration.
When the cycles of demineralisation and remineralisation are repeated, the outer parts of the tooth enamel may change over time and become more resistant to the acidic environment. This is due to the fact that the critical pH value of the newly formed crystals is lowered (e.g. down to pH 4.5).
A third mechanism by which fluoride helps maintain healthy teeth is its effect on oral bacteria - its antibacterial effect.
There are several bacteria that cause tooth decay. The most important of these is Streptococcus mutans.
Fluoride can act on bacterial cells. It inhibits their enzyme systems, affects the permeability of cell membranes or reduces the amount of acid produced by the bacteria.
In this case, we are therefore talking about an indirect effect on the demineralisation of dental tissues.
Role of fluoride in the body
The main sources of fluoride for the body are drinking water and food. The largest proportion of fluoride enters the body via the digestive tract.
However, fluoride can also enter the body through inhalation or skin contact.
The most common exposures to fluoride are from food intake, drinking, use of products containing fluoride compounds such as toothpaste, dyes, pesticides or metal or glass processing activities.
Absorption
Fluoride ingested in food or drinking water is absorbed relatively quickly and to a high degree in the digestive tract. Up to almost 90 % of the total amount of fluoride in ingested food is absorbed in the stomach (minority) and in the small intestine (majority).
Fluorine from ingested food reacts with the acidic content in the stomach. It is subsequently absorbed mainly as sodium fluoride, hydrogen fluoride or fluorosilicic acid.
The part of fluoride that is not absorbed in the gastrointestinal tract is excreted in the faeces (approximately 10 %).
Absorption of fluoride may be affected by additional concurrent food intake.
For example, calcium, aluminium or magnesium cause a significant reduction in the absorption of some fluoride compounds because they form insoluble and difficult-to-absorb complexes with fluoride.
Distribution
By absorption from the gastrointestinal tract, fluoride enters the bloodstream and is distributed by the blood to the necessary sites.
In the blood, fluoride binds to plasma proteins. It reaches its highest concentration in the blood approximately 20-60 minutes after ingestion.
The amount of fluorine in the body of an adult is approximately 3 mg. Almost all of it (99 %) is concentrated in hard mineralised tissues - bones and teeth. The remaining 1 % is deposited in soft tissues.
When fluoride intake is excessive, it begins to be deposited in larger amounts in soft tissues.
Several factors influence the total fluoride content of the body. These include acid-base balance, blood composition, hormonal activity, kidney function, genetic factors, diet, physical activity and even altitude.
Fluoride is also able to cross the placenta. The amount that passes through the placenta depends on the amount of fluoride in the mother's blood. The higher the amount, the higher the proportion of fluoride in the placenta.
The concentration in the placenta is approximately 60% of the total concentration of fluoride in the mother's blood.
If the concentration of fluoride in the mother's blood increases significantly, the placenta can act as a barrier. It prevents the passage of excessive amounts of fluoride to the fetus, thus protecting it from high concentrations.
Fluoride also passes into breast milk in small amounts.
Excretion
Fluoride is mainly excreted from the body through the kidneys. This means that it is excreted in the urine.
Since the concentration of fluoride in the blood is not regulated by the process of homeostasis, the kidneys are the main organ responsible for regulating and maintaining physiological levels of fluoride in the human body.
Diseases or various disorders of kidney function result in the retention of fluoride in the body and thus in an increase in fluoride levels.
An insignificant proportion of fluoride is also removed by sweat, saliva or faeces.
What is the recommended daily intake of fluoride?
Recommendations for the average daily intake of fluoride have not been established due to a lack of data.
However, the European Food Safety Authority publishes values for an adequate intake of fluoride. An adequate intake is an average value based on observation. It is assumed to be adequate for the needs of the population.
In addition, there is also an upper limit of fluoride intake that is still tolerable for humans. This limit represents the maximum long-term daily intake of fluoride from all sources at which there is no risk of adverse health effects.
Tabular summary of adequate daily intake and upper limit of fluoride intake by age
| Age group | Adequate intake of fluoride | Upper limit of fluoride intake |
| Infants (aged 7-11 months) | 0.4 mg/day | Not applicable |
| Children aged 1-3 years | 0.6 mg/day | 1.5 mg/day |
| Children aged 4-6 years | 1 mg/day (boys) 0.9 mg/day (girls) | 2.5 mg/day |
| Children aged 7-8 years | 1.5 mg/day (boys) 1.4 mg/day (girls) | 2.5 mg/day |
| Children aged 9-10 years | 1.5 mg/day (boys) 1.4 mg/day (girls) | 5 mg/day |
| Adolescents aged 11-14 years | 2.2 mg/day (boys) 2.3 mg/day (girls) | 5 mg/day |
| Adolescents aged 15-17 years | 3.2 mg/day (boys) 2.8 mg/day (girls) | 7 mg/day |
| Adults (aged ≥ 18 years) | 3.4 mg/day (boys) 2.9 mg/day (females) | 7 mg/day |
| Pregnant women (aged ≥ 18 years) | 2.9 mg/day | 7 mg/day |
| Breastfeeding women (aged ≥ 18 years) | 2.9 mg/day | 7 mg/day |
Food and other sources of fluoride
Although fluoride is an important part of our daily lives, we consume only relatively small amounts of it daily.
Drinking water is the source of most of the fluoride for our bodies. Fluoride is naturally present in drinking water. Nowadays, the concentration of fluoride in water is deliberately increased by adding fluoride. This is called fluoridation of water.
In addition to drinking water, the total amount of fluoride a person ingests during the day also includes fractions from foods or other products they use daily.
The fluoride content of food is usually low (less than 0.05 mg/100 g) and only 0.3-0.6 mg contributes to the total daily fluoride intake.
Foods richer in fluoride include, for example, teas, minced chicken containing ground bones, canned meat, sea fish (especially if eaten with bones, e.g. sardines), cereals, fruit juices (especially grape juice), milk or baby food.
Among plants, the tea tree (Chinese tea tree) is a good source of fluoride. Fluoride is mainly concentrated in its leaves. The more acidic the soil on which the plant grows, the more fluoride accumulates in it.
Consumption of medicines, dietary supplements, the use of fluoride toothpastes or other oral hygiene products (mouthwashes, foams, gels, varnishes, professional dental products, etc.) also contribute to the total daily intake of fluoride.
Foods that can potentially interfere with fluoride levels in the body include, for example, chlorides, which are present in particular in table salt. Low intakes of chlorides reduce the rate of excretion of fluoride by the kidneys and thus increase its retention in the body.
In addition, a diet rich in meat protein causes more fluoride to be retained.
The aforementioned calcium, aluminium or magnesium compounds are also responsible for a significant reduction in fluoride absorption.
Fluoridation of water and food - what is the significance?
Fluoridation of water or other foods is the process of deliberately adding fluoride in controlled amounts to increase its concentration in these products.
The aim of this measure is to ensure a systematic intake of fluoride in the population without the need to actively control this intake. It is also an attempt to ensure that fluoride is taken in at the levels necessary to maintain health and prevent the health consequences of any deficiency.
Water fluoridation was first introduced in 1945 in the USA and is still practised in many countries around the world.
The introduction of water fluoridation has led to a significant reduction in the prevalence of dental caries in the population, both in milk teeth and permanent teeth. It is therefore an effective preventive measure against dental caries in children and adults.
It is important that when fluoridating water, the level of fluoride is not exceeded to the extent that toxicity and side effects occur.
Therefore, the optimal concentration of fluoride in drinking water is set between 0.8 and 1.5 mg/l (in Europe).
In addition to water fluoridation, alternative methods are also used, such as adding fluoride to milk or table salt.
These methods are used to a lesser extent, mainly in areas where dental services are limited or where fluoridation of public water is not possible.
Fluoridation has long been the subject of much controversy, particularly because of its association with the occurrence of negative effects on the human body. Over the years it has found many opponents.
Some studies have shown that excessive fluoride intake by children contributes to negative effects on their brain development. For this reason, too, fluoridation must strictly adhere to the concentration limits set.
Fluoride in toothpastes or as a dietary supplement
The success of water fluoridation in reducing the incidence of dental caries and slowing the progression of existing carious lesions has led to the development of many products containing fluoride.
These include food supplements, toothpastes, mouthwashes or professional dental products such as foams, gels, varnishes and others.
The first toothpaste containing fluoride, specifically sodium fluoride, was produced in 1955.
These products also contribute significantly to the total amount of fluoride that is taken into the body on a daily basis.
Their simultaneous use together with the intake of fluoridated water therefore raises concerns in terms of exceeding the daily permissible intake limits.
Children are particularly at risk in this respect.
Children are at increased risk of swallowing toothpaste when brushing their teeth. It is estimated that children under the age of 6 years swallow approximately 0.3 mg of fluoride from toothpaste each time they brush their teeth.
It is therefore recommended that parents supervise their children when brushing their teeth.
Toothpastes with a lower fluoride content should be used. A small amount should be applied to the toothbrush, about the size of a grain of rice for young children under 3 years of age and the size of a pea for older children aged 3 to 6 years.
The use of fluoride supplements is usually recommended for children at high risk of tooth decay or as an alternative when only non-fluoridated water is available.
Currently, fluoride is only available on the market as part of multi-ingredient products - multivitamin or mineral supplements.

How does fluoride deficiency and excess manifest itself?
When fluoride deficiency is severe or prolonged, fluoride levels in the body drop.
So far, the only known consequence of this deficiency is an increased risk of tooth decay in people of any age.
Conversely, high fluoride levels are much more commonly encountered.
High levels of fluoride are more common.
Excess fluoride in the body is caused by the intake of high doses of fluoride, most often from an uncontrolled combination of different sources - drinking water, dietary supplements, toothpaste and oral hygiene products.
Elevated levels of fluoride are dangerous to the body. It causes a range of adverse symptoms and can lead to toxicity.
Children and persons with known hypersensitivity to fluoride and its compounds are at risk of toxicity.
Up to 80% of cases of fluoride toxicity are observed in children under 6 years of age due to ingestion of toothpaste or other oral hygiene products.
The lowest dose of fluoride at which acute adverse effects can already be observed is 5 mg/kg body weight.
The most common symptoms of acute toxicity include:
- Excessive salivation
- Nausea and vomiting
- Abdominal pain
- Diarrhoea
- Shallow breathing and weak heartbeat
- Sweating
- General weakness and trembling
- Cramps
The adverse effects of fluoride on the digestive tract are due to the formation and action of hydrofluoric acid.
Headaches, fatigue, itching, weakness and numbness of the limbs occur less frequently. In severe poisoning, tissue damage, respiratory and cardiac problems occur.
A blood fluoride level of 9.1 mg/l is considered no longer compatible with life.
In addition to the above symptoms, elevated fluoride levels cause a number of other disorders that are not usually visible to the eye.
In the blood, the released fluoride ions combine with calcium and in significant excess cause a drop in calcium levels - hypocalcemia.
In high doses, fluoride stimulates the function of osteoblasts (cells that break down bone tissue) and in turn inhibits the function of osteoclasts (cells that build bone tissue).
It also causes a slowing down of many enzyme systems.
Chronic, i.e. long-term intake of high doses of fluoride into the body leads to joint pain, thickening and increased bone density.
Fluorosis as a serious consequence of excessive fluoride
The most serious consequence of chronic fluoride overdose is the development of dental fluorosis.
It is a developmental disorder of tooth enamel that occurs during the period of tooth enamel formation and is caused by excessive systemic exposure to fluoride during the first six to eight years of life.
Thus, dental fluorosis affects children. Once tooth development is complete, fluorosis no longer develops even with high levels of fluoride in the body.
The affected enamel contains more protein, is more porous and less transparent compared to healthy teeth.
The initial form of dental fluorosis is manifested by the appearance of small opaque spots or stains on the enamel.
In the more advanced to severe form, the stains are larger and more pronounced, and are coloured yellow or light brown. Narrow white horizontal lines run through the teeth. The enamel is distorted, porous and even lost.
In the case of deciduous dentition, fluorosis is most often found on the molars or the eye teeth. In the case of permanent dentition, it occurs on the molars and the front teeth.
Therefore, dental fluorosis is in a way an aesthetic problem.
Severe cases of chronic fluoride overdose can also lead to bone fluorosis. This develops over a period of years.
It is characterised by changes in bone structure. Excessive amounts of non-mineralised bone tissue are formed and bone mineralisation is also impaired.
In the early stages, bone density increases. However, the bones are fragile and break easily.
The disease progresses over the years to joint pain and stiffness, muscle weakness, calcification of ligaments and tendons, and even loss of mobility or nerve problems.
Finally, we can mention the side effects caused by fluoride inhaled or in contact with the skin.
These include irritation of the mucous membranes of the respiratory tract, eyes and skin, and possibly the development of liver and kidney disorders.
Interesting articles on health:
Interesting resources
Related