- pubchem.ncbi.nlm.nih.gov - Iodine
- ncbi.nlm.nih.gov - Iodine, Iodine metabolism and Iodine deficiency disorders revisited, Farhana Ahad and Shaiq A. Ganie
- sciencedirect.com - IODINE Properties and Determination, M.R.L'Abbé
- sciencedirect.com - IODINE Physiology, J.A.T.Pennington
- ncbi.nlm.nih.gov - Health Consequences of Iodine Deficiency, Umesh Kapil
- pubmed.ncbi.nlm.nih.gov - Iodine excess, Hans Bürgi
- multimedia.efsa.europa.eu - Dietary Reference Values for the EU
- szu.cz - Iodine and the thyroid gland, prof. MUDr. Václav Zamrazil, DrSc., RNDr. Jarmila Čeřovská, CSc.
- solen.sk - Thyreopathies in the general practitioner's outpatient clinic, Doc. MUDr. Soňa Kiňová, PhD., MUDr. Michal Koreň, PhD
Why is iodine important in our diet? How does it affect the body?
Iodine is an essential micronutrient that plays a key and irreplaceable role in the regulation of energy metabolism in all of us. What are its other functions? Why is insufficient iodine intake still a looming problem? What are the consequences of deviations from normal iodine levels in the body?
Article content
- What do we know about iodine?
- What is the biological function of iodine?
- Iodine - from uptake to excretion
- Do you know the sources of dietary iodine?
- What is the recommended daily intake of iodine?
- Deficiency versus excess iodine in the body
- What causes iodine deficiency?
- What causes excessive iodine intake?
What do we know about iodine?
Iodine is a non-metallic chemical element that has the chemical symbol I. It is derived from the Latin word iodium. Its origin is in the Greek word iodes, which translates as purple.
The name refers to its appearance. Iodine vapour is purple in colour.
Iodine is an element of group 17 of the periodic table of chemical elements and is found in the 5th period.
It belongs to a group of elements called halogens, which also includes fluorine, chlorine, and bromine. The group was named for the ability of its elements to form salts (from the Greek hals - sol, gennaó - I form).
Among the halogens, it is the most electronegative element, with the lowest electronegativity and also the weakest oxidizing ability. Its abundance is also the lowest compared to the other halogens.
Iodine was discovered by the French chemist Bernard Courtois in 1811.
When he isolated sodium and potassium compounds from the ashes of seaweed (which were subsequently used to make gunpowder), the accidental addition of more sulfuric acid raised a cloud of purple vapor from the ashes.
Courtois thought it was a new element but did not have the means to investigate it further.
Proof that it was a new element was eventually provided in 1814 by the French chemist Joseph Louis Gay-Lussac, who also gave it its name.
Iodine is a solid crystalline substance of a blue-black colour with a metallic lustre. It can be ground to a fine powder. It is slightly soluble in water. In organic solvents, on the other hand, it dissolves readily to form purple, pink or brown solutions.
Under normal conditions, i.e. under standard pressure and temperature, iodine sublimes relatively easily as a purple vapour with an irritating odour. The vapour is made up of diatomic iodine molecules - I2.
A tabular summary of basic chemical and physical information about iodine
| Name | Iodine |
| Latin name | Iodium |
| Chemical name | I |
| Classification of elements | Halogen |
| Grouping | Solid (at room temperature) |
| Proton number | 53 |
| Atomic mass | 126,904 |
| Oxidation number | -1, +1, +3, +5, +7 |
| Melting point | 113,7 °C (in l2 form) |
| Boiling point | 184,3 °C (in l2 form) |
| Density | 4,93 g/cm3 |
Iodine is one of the least abundant non-metallic elements on the Earth's surface. It is mostly found in rocks, soil, water, plants, but also in animal tissues - in the form of iodides and iodates.
Rare iodine-containing minerals are, for example, lautarite or dietzeit.
The highest amounts of iodine are found in seaweed, sponges or coral, as well as in groundwater. Seawater contains the highest total amount of iodine due to leaching from rocks and soil.
Iodine escapes from seawater into the atmosphere through solar radiation and returns to the soil through precipitation.
The iodine content of groundwater is much lower than that of seawater and always reflects the iodine content of the surrounding soil.
The presence of iodine in soil is also variable, being highest in coastal areas and lowest in inland areas.
TIP: The thyroid gland: what are the symptoms of reduced or increased function?
Plants, crops or animal products usually reflect the iodine content of the soil. The higher the iodine content of the local soil, the higher the iodine content of the plants and subsequently the animal products.
Iodine and its compounds are mainly used as catalysts, stabilisers, dyes or pigments.
They are also included in animal feed additives, pharmaceutical products or disinfectants (iodine tincture). They are also used as a non-toxic contrast agent in radiological examinations.
Extra care should be taken when handling iodine. It can irritate or burn the skin or cause damage to the eyes and mucous membranes. Internal ingestion of iodine in elemental form is toxic.
What is the biological function of iodine?
Iodine has an irreplaceable function in the human body.
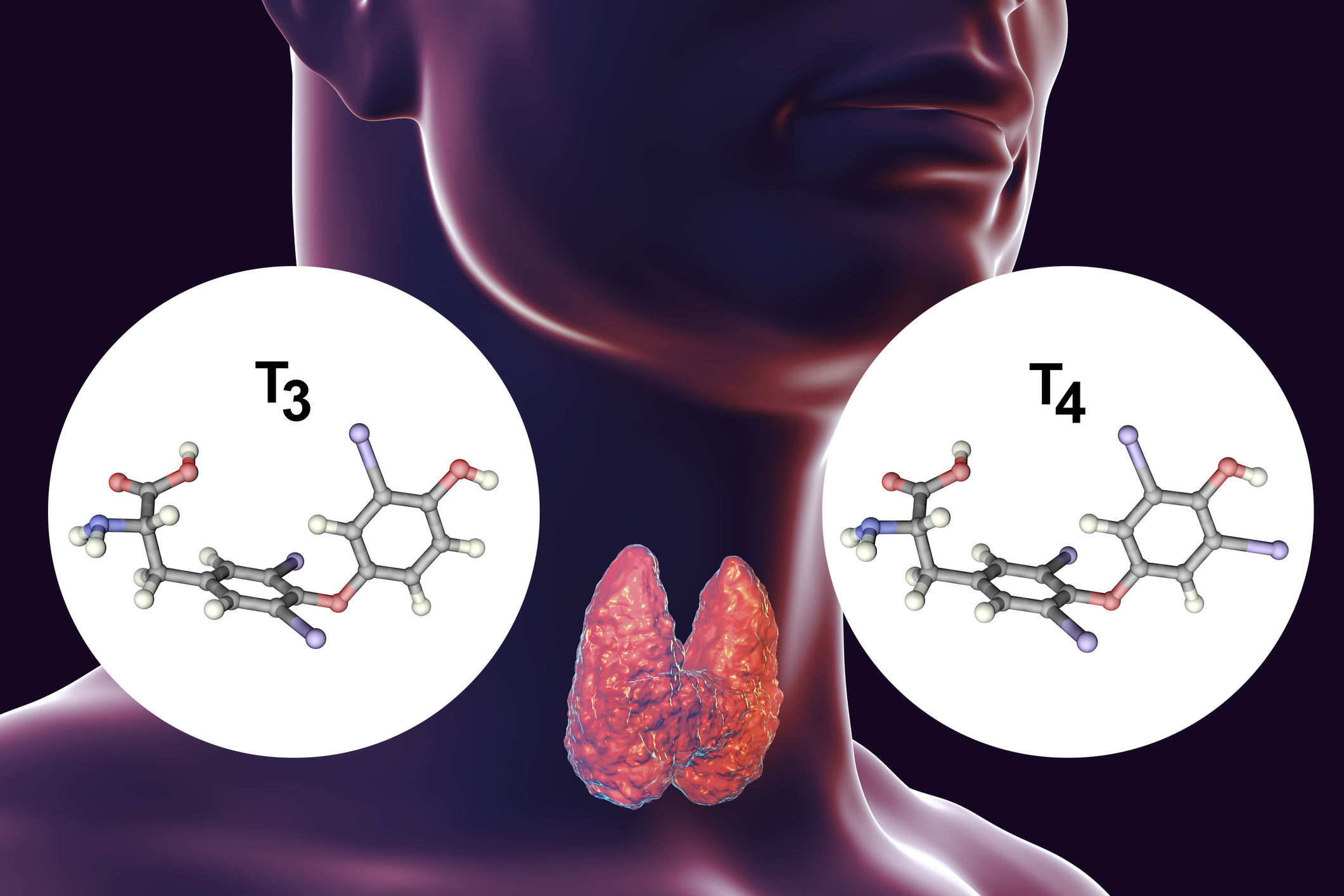
It is an essential nutritional element that is necessary for the production of the thyroid hormones thyroxine (T4) and triiodothyronine (T3).
In both cases, these are molecules of the amino acid tyrosine, to which iodine atoms are bound. In the case of thyroxine, these are four iodine atoms, in the case of triiodothyronine, three iodine atoms.
The thyroid gland is the largest endocrine gland in the body. Its job is to produce sufficient amounts of the above hormones, which are also called thyroid hormones.
Thyroxine is produced to a greater extent compared to triiodothyronine. It is considered a prohormone. It is not hormonally active itself and represents a circulating supply for the production of already active triiodothyronine.
Thyroid hormones are involved in several biological functions in the body, which may therefore be related to iodine itself.
- They are important for the normal growth and development of the body (from the intrauterine phase to puberty).
- Throughout life, they strongly influence energy metabolism.
- They influence the development and function of the central nervous system.
- They ensure normal mental function and brain performance.
- Regulate homeostasis function, including energy and heat production.
- They affect performance and quality of life.
- Involved in the regulation of body weight.
- Reduce blood cholesterol levels.
- They increase the absorption of sugars in the digestive tract, the breakdown of fats and fatty acids.
- They regulate the use of oxygen in the cells.
The free form of iodine, i.e. without binding to the amino acid tyrosine, is unlikely to play a significant role in the regulation of metabolism.
Iodine - from uptake to excretion
The human body is unable to synthesize iodine on its own. It is therefore dependent on its intake in the diet or in the form of drugs and supplements.
Absorption
Iodine is largely taken in through food or drinking water. In these, it is found in various chemical forms.
In food, iodine is mainly found in the form of inorganic iodide I-, which is an easily absorbed form of iodine. Absorption occurs in the stomach or duodenum.
Other forms, such as iodate, must be reduced to iodide in the intestinal environment before absorption.
Iodides are rapidly and almost completely absorbed into the blood in the digestive tract. In healthy people, absorption is more than 90 % of the amount ingested.
The absorption of iodides may be influenced by the composition of the concurrent food intake, e.g. calcium, magnesium, iron, fluoride, nitrate or thiocyanate.
Distribution
The total blood concentration of iodine ranges from approximately 40 to 80 µg/l. It includes both inorganic iodine and bound iodine (e.g. in the form of thyroid hormones).
Concentrations may be elevated in cases of excessive iodine intake or pathologically elevated thyroid function.
Circulating iodine in the bloodstream is taken up primarily by the thyroid gland and kidneys.
If the body has an adequate iodine intake, the thyroid gland does not use more than 10 % of the iodine absorbed. In the case of a prolonged insufficient iodine intake, the proportion of iodine taken up from the blood is higher than 80 %.
In addition, iodine is also taken up in small amounts by the salivary glands, the lining of the stomach and is found in the eyes and cervix. The function of iodine in these parts of the body is still unknown.
It is important to mention that iodine is also taken up by the mammary glands in the case of breastfeeding mothers. Iodine plays an important role in the development of newborn babies.
Thyroid hormones circulating in the blood are predominantly bound to protein carriers. Only less than 1% are found in free form. Yet it is these free fractions that are responsible for the hormonal action.
In a healthy human body, 15-20 mg of iodine is present. Of this, 70-80% is found in the thyroid gland. This amount depends on iodine intake and decreases when iodine intake is reduced.
In the case of long-term iodine deficiency, the amount of iodine in the thyroid gland can drop to below 20 µg.
Metabolism and excretion
The metabolic process of iodine begins with its uptake by the thyroid gland. The thyroid gland uses the iodine it receives to produce the hormones thyroxine and triiodothyronine.
The lifetime of thyroxine (the time it circulates in the blood and performs its function) is 5-8 days. The lifetime of triiodothyronine is shorter, 1.5-3 days.
These hormones subsequently undergo degradation processes. During these processes, iodine is released from the hormone molecule, which is still present in the blood plasma.
The degradation processes of thyroid hormones are dependent on selenium intake, as selenium is an essential component of the enzymes involved in degradation.
Iodine released into the blood can either be reabsorbed by the thyroid gland or excreted from the body.
Iodine is mainly excreted from the body via the kidneys. More than 90% of the iodine taken in from the diet is eventually excreted in the urine.
Smaller amounts of iodine are excreted through faeces, perspiration and, in the case of breastfeeding mothers, breast milk.
Do you know the sources of dietary iodine?
The natural occurrence of iodine in food is highly variable. This variability is due to the fact that soil and water have different concentrations of iodine in different parts of the world.
Soils with the highest iodine content are found in coastal areas, while soils with the lowest iodine content are found in inland and mountainous areas.
The higher the iodine content of soil and water, the higher the iodine content of local plants and crops.
In the case of animal products, the differences in iodine content are due to the nature of the feed consumed by the animals concerned.
The iodine concentration in milk is usually higher in winter because animals are fed more iodine supplements then.
In addition, the iodine content in plants is inadvertently increased by fertilisers or treatments and in animal products by the addition of food additives or dyes.
In most cases, plant foods have a lower iodine content than animal foods because of the low concentration of iodine in the soil (except in coastal areas).
Seaweed has the highest iodine content.
Foods rich in iodine are seafood, green and leafy vegetables (e.g. spinach), milk, meat, eggs, cereals.

Iodine deficiency in the diet and related thyroid diseases have been, and to some extent still are, a worldwide problem.
This is being addressed by deliberately adding iodine to foods. We are talking about the process of fortification.
Probably the best known example of deliberate addition of iodine to food is the addition of iodine to table salt (iodised salt). This occurs mainly in areas where the soil and water are poor in iodine.
To increase the content, iodine is also added in the form of iodate to dough (bread, cakes) or as the red food colouring erythrosine to sweets or cereals.
In some countries, iodine is also added to other commonly used foods such as rice, tea or oils.
Other sources of iodine besides food can be medicines, mineral supplements or supplements with extracts from seaweed, plants or fish. Also radiological contrast agents, skin disinfectants or water purification tablets.
Examples of iodine-containing medicines include amiodarone, a drug used to correct irregular heart rhythms. They also include dietary supplements containing potassium - in the form of KI or KIO3.
In the context of food, it is important to mention substances that counteract thyroid hormones in the body. We are talking about antithyroid substances or also strumigene.
These substances reduce the production or use of thyroid hormones. Examples are thiocyanates, which are found in cabbage, kale, kohlrabi, cauliflower, broccoli or forage.
What is the recommended daily intake of iodine?
Recommendations for the average daily intake of iodine have not been established due to the lack of data.
However, the European Food Safety Authority publishes values for adequate iodine intake. Adequate intake is an average value based on observation. It is assumed to correspond to the needs of the population.
Table of adequate daily intakes of iodine by age
| Age group | Adequate iodine intake |
| Infants (aged 7-11 months) | 70 µg/day |
| Children (aged 1-3 years) | 90 µg/day |
| Children (aged 4-6 years) | 90 µg/day |
| Children (aged 7-10 years) | 90 µg/day |
| Adolescents (aged 11-14 years) | 120 µg/day |
| Adolescents (aged 15-17 years) | 130 µg/day |
| Adults (age = 18 years) | 150 µg/day |
| Pregnant women (age = 18 years) | 200 µg/day |
| Breastfeeding women (age = 18 years) | 200 µg/day |
Deficiency versus excess iodine in the body
With a deficiency, but also with an excessive intake of iodine, the body can develop disorders or diseases that in some cases are really serious.
A reliable indicator of the body's iodine supply is a urine test, the size of the thyroid gland and the amount of thyroid hormones.
The most important indicator is the urine iodine level. More than 90% of the iodine ingested in the diet is excreted in the urine, so this test is reliable.
It is optimal to determine iodine excretion over a 24-hour period, as the concentration of iodine in the urine fluctuates during the day.
Table of urinary iodine values and their significance
| Urinary iodine value | Rate of iodine uptake into the body |
| < 19 µg/l | Severe iodine deficiency |
| 20-49 µg/l | Moderate iodine deficiency |
| 50-99 µg/l | Mild iodine deficiency |
| 100-199 µg/l | Optimal supply |
| 200-299 µg/l | Increased iodine content |
| 300-499 µg/l | Excessive iodine content |
| > 500 µg/l | Significantly high iodine content |
The size of the thyroid gland as an organ is closely related to iodine intake. Changes in its size may indicate both insufficient and excessive iodine intake.
Determination of thyroglobulin, thyrotropin and, in rare cases, thyroid hormone levels can also provide information on the body's iodine supply.
Thyroglobulin is the precursor of thyroxine and triiodothyronine, i.e. it is the origin of these hormones. Thyrotropin is also a hormone, it is formed in the brain and stimulates the production and secretion of thyroxine and triiodothyronine.
Comparing the prevalence of iodine deficiency and excess disorders, iodine deficiency disorders predominate.
What causes iodine deficiency?
Iodine deficiency diseases are among the most serious pandemics worldwide, affecting virtually all continents.
Iodine deficiency remains one of the most important but preventable causes of brain damage and mental retardation.
The most important function of iodine is to participate in the synthesis of thyroid hormones. In case of its deficiency, the production of hormones automatically decreases.
In case of short-term iodine deficiency, the thyroid gland can compensate for this deficiency by using its pre-existing hormone stores.
The thyroid gland has a built up supply of thyroid hormones for several months in advance. This is to avoid disturbances in their production in cases where iodine is unavailable in the human diet in the short term.
If the deficiency in iodine intake persists, the supply of hormones is depleted and their blood levels drop. In response to this condition, the secretion of thyrotropin increases in the brain. Thyrotropin attempts to act on the thyroid gland to increase the production of hormones, thereby compensating for their reduced levels.
However, even under the pressure of thyrotropin, the thyroid gland is unable to produce thyroxine and triiodothyronine due to iodine deficiency.
Instead, the thyroid gland enlarges, in some cases to enormous proportions.
Adequate iodine intake is especially essential during the period of human growth and development, whether we are talking about newborns, children or adolescents. It is also important during pregnancy, the period of fetal development.
A deficiency leads to a slowdown in the development, growth and maturation of organs and tissues. Different tissues are differently sensitive to deficiency. The most sensitive organ is the brain.
The critical period is the time from the second trimester to the second year of the child's life. Even a mild iodine deficiency during this period can lead to serious and permanent brain damage.
In mild forms of iodine deficiency, in addition to goiter, mild developmental disorders occur, especially in fetuses, children and adolescents. These include a decrease in the intelligence of the individual concerned and the occurrence of hyperactive child syndrome.
The adverse effect of iodine deficiency on sexual development and associated infertility is also discussed.
Moderate forms of deficiency lead to goiter and even hypothyroidism. This is a reduced thyroid function.
In severe iodine deficiency in fetuses, newborns and children, severe developmental disorders occur. The individual is severely and permanently affected. We speak of endemic cretinism, which is already an extreme manifestation of iodine deficiency.
Cretinism is characterised by disturbances in bone development leading to deformities of the body and face. It is also manifested by a significantly reduced intellect, which makes it impossible for the sufferer to exist independently.
Tabular overview of the health consequences of iodine deficiency in humans by age
| Age group | Consequences of iodine deficiency |
| Pregnancy and fetal development |
|
| Newborns |
|
| Children and adolescents |
|
| Adults |
|
| Elderly |
|
The options for preventing and dealing with the consequences of iodine deficiency are relatively simple. It is necessary to increase iodine intake in the diet or through dietary supplements.
What causes excessive iodine intake?
Unlike iodine deficiency, the adverse effects of excessive iodine intake are seen in a smaller proportion of the population, up to 10%.
Some individuals can tolerate very high levels of iodine without visible side effects.
The explanation for this difference probably lies in the fact that the thyroid gland has sufficient regulatory mechanisms. It can thus adapt to excess iodine.
The more sensitive population group that reacts adversely to excess iodine are people with usually low iodine intake, thyroid disorders or increased sensitivity to iodine.
The most common symptoms of iodine hypersensitivity are hot flashes, swollen salivary glands, vision problems, skin problems such as hives or rashes.
In the case of excessive iodine intake into the body, it is very important to distinguish whether it is a short-term excess of iodine or a long-term increase.
In the first case, the changes are most often caused by the administration of certain drugs or dietary supplements with a high iodine content (e.g. amiodarone).
An even greater iodine load occurs in examinations where iodine is used as a contrast agent - X-ray examinations, computed tomography.
Short-term but very intense iodine loading can cause thyroid dysfunction and activation of autoimmune reactions.
From a health point of view, a long-term increase in the content of iodine in the diet is more dangerous and serious. Most often this happens when dealing with iodine deficiency.
We speak of excessive iodine in the body when the level of iodine in the urine exceeds 300 µg/l. Levels that are already considered dangerous for humans are higher than 500 µg/l.
What are the main risks of long-term excessive iodine intake and what health problems does it cause?
- Hyperthyroidism - Increased thyroid function
- Autoimmune or inflammatory diseases of the thyroid gland
- Goiter
- Hypothyroidism - Thyroid function and hormone production is paradoxically reduced in the short term with a high iodine load, the reduction may then persist in people with pre-existing thyroid disease.
- Inflammatory thyroid disease in children under one year of age if their mother was deficient in thyroid hormones before pregnancy
- In more severe cases, probably also thyroid tumours
Interesting resources
Related










