- training.seer.cancer.gov - Introduction to Biological Therapy
- topdoctors.co.uk - What is biological therapy?
- medicinenet.com - Biological Therapy, Melissa Conrad Stöppler, MD, Jerry R. Balentine, DO, FACEP
- who.int - Vaccines and immunization
- oncologynurseadvisor.com - Biological Therapies for Cancer (Fact Sheet)
- archive.bio.org - How Do Drugs and Biologics Differ?
- iapo.org.uk - Introduction to Biologics
- ncbi.nlm.nih.gov - Defining the difference: What Makes Biologics Unique, Thomas Morrow, MD
- ncbi.nlm.nih.gov - Biologic therapies: what and when?, Sarah L Johnston
- uspharmacist.com - Naming of Biological Products, Golden L. Peters, PharmD, BCPS, Erin K. Hennessey, PharmD, BCPS
- journals.lww.com - Injectable Biologics, Kubrova, Eva MD; D'Souza, Ryan S. MD; Hunt, Christine L. DO; Wang, Qian MD, PhD; van Wijnen, Andre J. PhD; Qu, Wenchun MD, MS, PhD
- solen.sk - Targeted biological therapy of the most common tumor diseases and its side effects, doc. MUDr. Peter Beržinec, CSc.Oncology Department, Specialized Hospital St. Svorad Zobor, n. o., Nitra
- solen.sk - Biological drugs from the perspective of pharmacy,PharmDr. Katarína Bruchatá, PhD., Mgr. Peter HeinzUniversity of Veterinary Medicine and Pharmacy in Košice, Institute of Pharmaceutical Chemistry, Košice
What is biological therapy, biological medicine? It helps where others fail

Are medicines made entirely from chemicals and in a laboratory environment? Biological medicines are the opposite. What is a biological medicine?
Article content
- What do we mean by biological therapy?
- What role does immunity play?
- Biological medicine - how does it differ from conventional medicine?
- The main differences between biological and chemical medicine
- What are the known structures of biologics?
- Nomenclature of biological medicinal products
- How is a biologic drug made?
- Can we expect side effects with biological treatments?
- Some examples of biological medicinal products and their uses
If you think medicines are made entirely from chemicals and in laboratory conditions, biologics may convince you otherwise. What is a biologic and how long has it been in our lives?
Let's take a look at the history...
In 1796, Edward Jenner, an English physician, considered the father of immunology, conducted an experiment based on his long-term observations. He collected fluid from the skin lesions of a milkmaid who was infected with cowpox. He deliberately administered it (inoculated) to an eight-year-old boy named James.
The boy contracted cowpox with an easy course.
Two months later, Edward Jenner inoculated the boy again, this time with a secretion from the skin lesions caused by smallpox (variola). As expected, James did not develop smallpox.
Smallpox was a widespread, highly contagious and fatal disease before the invention of vaccination and was the scourge of mankind for several centuries (statistically responsible for 8-20% of all deaths in Europe).
The smallpox vaccine was the world's first vaccine.
One hundred years later, American surgeon William Coley noticed an interesting connection in a German immigrant named Fred, who had an inoperable malignant tumor on his neck. After Fred was diagnosed with a skin infection caused by a bacterium of the genus Streptococcus, his tumor cells disappeared.
William Coley hypothesized that the body's response to infection must have some effect on the tumor.
He continued his research. He first gave live bacteria to patients with certain types of cancer, then only their toxins (infected patients). This led to tumour remission in some cases, i.e. a reduction in signs and symptoms.
This treatment was known for many years as Coley's toxins.
What do these two stories from history have in common?
In both cases it is immunization. The process by which our body's immune system develops a natural protective barrier and becomes resistant (immune) to the action of foreign substances. This reduces the risk of infection and disease.
Vaccines (inoculants) are a key tool for the immunisation process.
It was with the discovery of immunisation more than 200 years ago that biological medicine came to the fore.
Today, biological therapy is a rapidly developing area of modern medicine.
Thanks to significant advances in the understanding and knowledge of the processes taking place in the human body, whether we are talking about natural or disease processes, it is being applied in many medical fields. At the same time, it is becoming an increasingly common and effective form of therapy for a wide range of human diseases.
What do we mean by biological therapy?
At first glance, the phrase "biological treatment" or the word "biological" may evoke the current fashionable meaning of "natural and chemical-free".
However, let us not confuse biological treatment with herbal medicines. The meaning of the word 'biological' in the case of these medicines refers to the fact that living organisms are used to produce them.
The principle of biological medicine is to use the body's natural immune system to fight disease or infection.
Its effect can be harnessed in several therapeutic directions, depending on the way in which the treatment is able to influence the biological processes in the body.
In what way it acts on the organism:
- It may involve stimulating certain components of the immune system in order to treat disease, inflammation or tumors.
- Conversely, biologics can also be used to suppress the immune system. This is particularly used in transplantation (preventing transplant rejection) or in the treatment of autoimmune diseases.
- Use of biologics to protect the body from the side effects of other concomitant treatments.
- Use in targeted therapy - in this case, the biologic is used to promote cell growth or to kill cells (e.g. cancer cells) in a targeted manner by affecting specific molecules needed for cell growth and proliferation.
In general, there are two basic types of biological treatment.
The first is immunotherapy, which uses various methods or drugs to influence the immune system. The immune system can thus create an inhospitable environment for the existence or growth of, for example, cancer cells.
The second type is cytotoxic therapy, also called cell-killing therapy. This type of treatment uses proteins called cytotoxins that are produced by the body's cells. Cytotoxins attack foreign cells and kill them directly. In some cases, they can inhibit the growth and multiplication of these cells.
Thus, we can summarise that biological therapy is most often used in oncology to treat various types of cancer, and in rheumatology to treat autoimmune or genetic diseases.
Biological therapy is more often used when other treatments (e.g. chemical drugs) are not effective or not available. However, it is also increasingly used as a first choice treatment, mainly due to its highly specific effect.
What role does immunity play?
The immune system is a complex network of organs, tissues and specific cells. It is able to recognise and destroy foreign substances such as bacteria or viruses, but also damaged, infected and abnormal cells in the body.
It can also remember the attacker, so that it reacts more quickly the next time it encounters him than the first.
The moment the immune system recognizes a foreign substance, called an antigen, a series of processes called the immune response is triggered.
The main players in the immune response are white blood cells (leukocytes). Each leukocyte has a specific type of action.
The following table gives examples of leukocytes and their main roles
| A subset of white blood cells | Representatives | Roles |
| Lymphocytes | T-lymphocytes | Directly attack foreign, infected, or tumor cells, sending a signal and activating other protective components of immunity. |
| B-lymphocytes | Produce antibodies that recognize and attack foreign substances - antigens. | |
| NK cells | Produce potent chemicals that bind to foreign substances and destroy them (even without first meeting them). | |
| Monocytes | Macrophages | Monocytes are rapidly transported into the affected tissue and differentiate into macrophages. The main role of macrophages is phagocytosis of foreign substances. |
| Dendritic cells | They support the activity of T-lymphocytes and B-lymphocytes. |
Biological medicine - how does it differ from conventional medicine?
Biological treatment is carried out with the help of biological drugs. These are characterized by the fact that living organisms, substances isolated from living organisms or substances produced by living organisms are used for their synthesis.
One of the earliest biologics was insulin.
The precursors to today's more modern biologics production processes were costly and uneconomical. Nearly two tonnes of pig pancreas were needed to produce just one small vial of insulin.
The adjective "biologic" thus derives from the fact that these drugs are of natural origin.
They can be substances derived from micro-organisms, plants or animals, but we are also talking, for example, about cells or tissues of human origin.
These substances then undergo treatment using various biotechnological processes to give them specific properties. For most biological medicines, the recombinant DNA method is used.
The nature and properties of a biological drug are crucial to its effectiveness in treating a given disease.
From a chemical point of view, a biologic is a very large, complex and intricate molecule or mixture of molecules. Most often it is a protein. But it can also be a sugar, a nucleic acid, a hormone, an enzyme, a component of the blood or the aforementioned living entities (cells and tissues).
Depending on the nature and properties of the active substance, biologics may have different routes of administration.
Examples of routes of administration:
- Oral (by mouth) - a lesser used route of administration because there is a risk of degradation of the large molecule of the biologic in the gastrointestinal tract, leading to loss of effect
- Injection or infusion, e.g. intravenous (into a vein)
- Transdermally - through the skin
Biological treatments are usually prescribed by specialist doctors. Depending on the problem to be treated with the biologic, this may include oncologists, oncohaematologists, as well as rheumatologists and gastroenterologists.
The main differences between biological and chemical medicine
Biologics have a higher potential to trigger an immune response (compared to chemical drugs). This is because the molecule of a chemical drug is too small to be recognized by the immune system as a foreign invader.
In contrast, with biologics, the immune system can very quickly recognize a relatively large molecule and initiate an immune response.
Biologics can also more accurately mimic or interfere with natural processes in our bodies.
Therefore, they are used in cases where treatment with chemical drugs is not available or insufficient.
A tabular summary of the differences between biological and chemical medicines
| Biological drug | Chemical drug | |
| Example | Monoclonal antibodies (treatment of cancer and autoimmune diseases) | Acetylsalicylic acid (treatment of pain and inflammation) |
| Chemical structure |
|
|
| Molecular weight |
|
|
| Treatment options |
|
|
| Manufacturing |
|
|
| Stability |
|
|
| Manufacturing process |
|
|
| Sensitivity to changes in manufacturing |
|
|
| Control |
|
|
| Quantity of medicinal products manufactured |
|
|
Example of a biological medicinal product
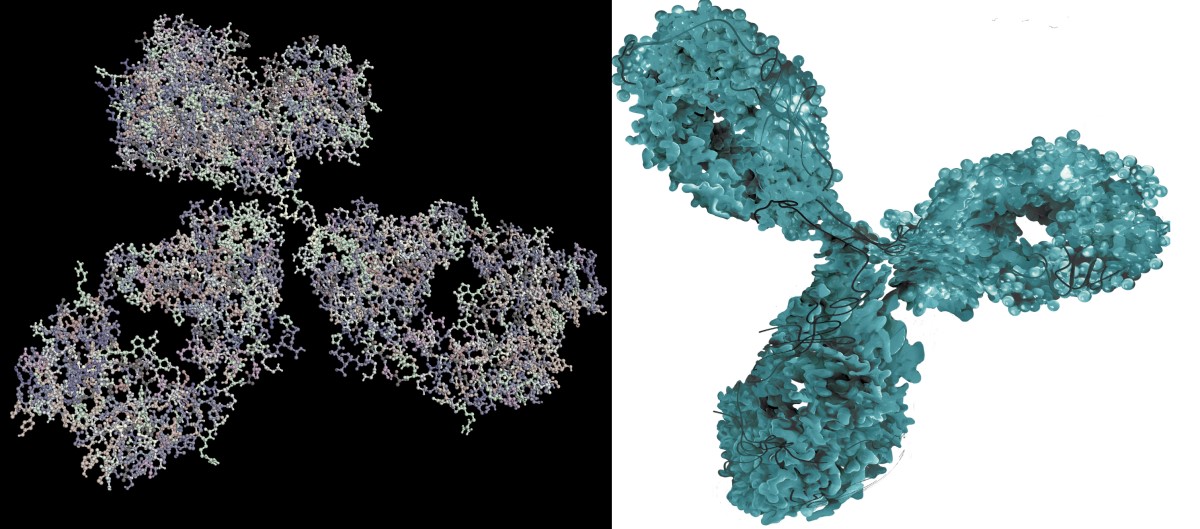
And an example of acetylsalicylic acid
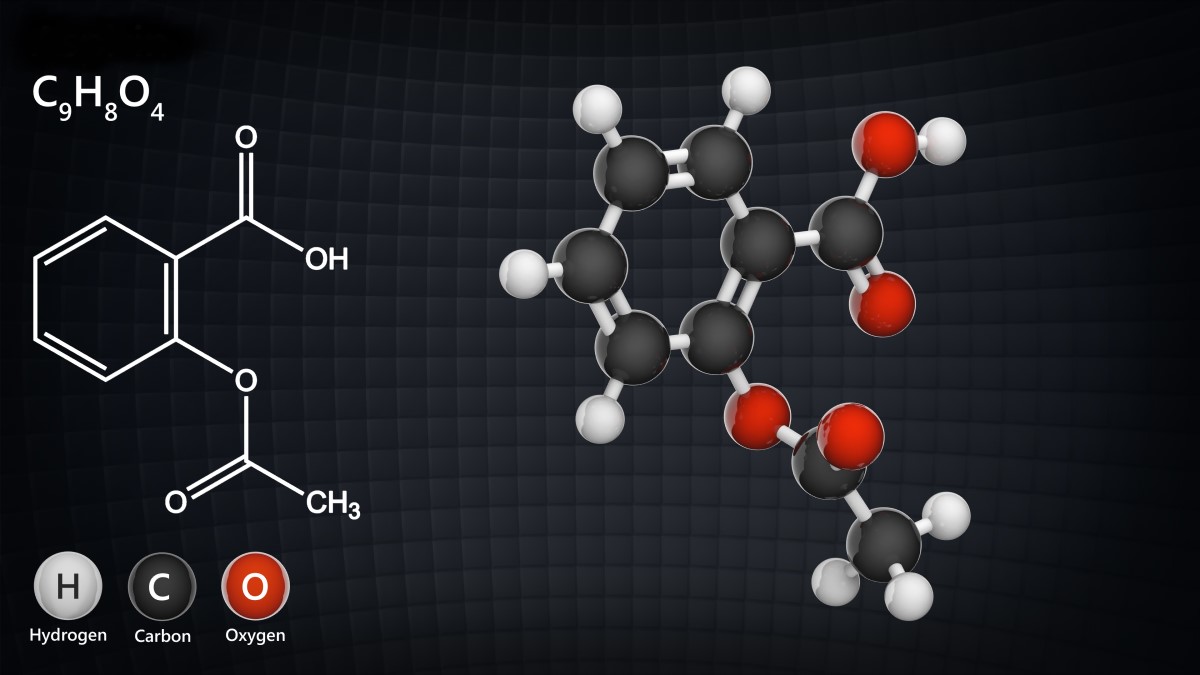
What are the known structures of biologics?
In terms of structure, there are different types of biologically active substances that are used in biological therapies.
In many cases, these are substances that are modified by recombinant DNA and have specific properties that are subsequently used in the treatment of diseases.
Hormones
For example, growth hormone, insulin, parathyroid hormone.
Chemicals that exhibit physiological activity. Most commonly peptides or steroids.
Interferons
Proteins produced by cells of the immune system in response to a viral infection or other stimulus.
They prevent viruses from replicating in the body.
Interleukins
Bioactive proteins produced by leukocytes, monocytes or other cells of the immune system.
One effect is to increase the activity of lymphocytes.
Growth factor
Promotes cell growth and maturation in particular.
It can be used to stimulate the bone marrow to produce cells or act as an anti-cancer agent.
Monoclonal antibodies
The most commonly used form of biological treatment.
These are laboratory synthesised substances. They mimic antibodies that are naturally produced by the immune system. They are able to recognise and bind to foreign particles - antigens.
Due to their wide range of applications, they are used in various fields of medicine - oncology, immunology, rheumatology, gastroenterology, etc. They can be used alone or in combination with conventional chemotherapy.
vaccines
Products containing antigens that are made from live, attenuated or killed micro-organisms, synthetic peptides or recombinant organisms.
They are given to prevent infections (serious and usually fatal) for which no other effective treatment is available.
Cancer vaccines
They form part of immunotherapy.
They stimulate the natural immune system to respond to cancer cells.
Gene therapy
Still an experimental form of treatment.
The principle is the insertion of genetic material (DNA or RNA) into living cells. The genetic material is introduced into the cells using a vector such as a virus.
Read more
Other structures:
- Polypeptides
- Proteins
- Blood and blood components
- Somatic (body) cells
- Tissues
Nomenclature of biological medicinal products
The names of biologics may seem complicated at first glance. However, the nomenclature defines rules that make it relatively easy to determine the structure, nature or use of a drug.
The rules for creating the names of medicinal products are based on the nomenclature classification approved by the United States Adopted Names Council (USANC). They must also be consistent with the World Health Organization's (WHO) INN programme, which assigns official names to medicinal products.
The United States Council on Adopted Names is a five-member board in the U.S. It reviews and approves the names of medicinal products to ensure that they are simple, informative, unique and that their names are logical in terms of pharmacology and chemical structure.
We are talking about so-called international nonproprietary names (INNs).
How do we know from the name of a biological medicinal product which substance it is in terms of structure?
It is important to look out for characteristic prefixes, suffixes or groups of letters in the middle of names. These can be used to navigate through the different groups of biological medicinal products or to find out what the product is used to treat.
Tabular overview of the nomenclature of biological medicinal products by chemical structure
| Type | Strain | Example of a medicinal product |
| Inhibitors | -nib | |
| angiogenesis inhibitor | -anib | pazopanib, nintedanib |
| tyrosine kinase inhibitor | -tinib | sunitinib, imatinib |
| Enzymes | -ase | lipase, amylase |
| Blood derivatives (erythropoietin type) | -poetin | epoetin |
| Growth hormone derivatives | som- | somapacitan |
| Drugs for the treatment of tumours | -ci- | bevacizumab |
| Monoclonal antibodies | -mab | |
| mice | -omab | blinatumomab |
| human | -umab | adalimumab |
| chimeric | -ximab | infliximab |
| humanized | -zumab | trastuzumab |
Tabular overview of the nomenclature of biological medicinal products by site of action
| Target structure | Strain | Example of medicinal product |
| Tumours | -tu(m)- | cetuximab |
| Cardiovascular system | -ci(r)- | bevacizumab |
| Bone | -o(s)- | denosumab |
| Immune system | -li(m)- | ipilimumab |
How is a biologic drug made?
The active ingredient of a biologic drug is part of a huge macromolecule whose structure is very often undefined. In terms of the molecules present, a biologic drug is heterogeneous (diverse).
The actual creation of a biologic product is preceded by research and development. In general, the research and development phase of a drug (even a chemical one) is an extremely demanding and long-term process. It is necessary to define a structure that has sufficient potential to become a future drug.
In order to obtain a new drug structure, it is necessary to discover the substance that will encode the synthesis of this structure. Most often, this substance is a gene or a protein.
This substance is then transferred into a suitable host organism (e.g. a bacterium or mammalian cell). The host organism starts to produce the substance of the desired structure.
The most commonly used host organism is the bacterium Escherichia coli or the yeast Saccharomyces boulardii.
All newly produced biological substances undergo a series of tests and evaluations to determine their ability to alter biological processes in the organism.
At the same time, their efficacy and safety are verified through preclinical (animal testing) and clinical studies (human testing).

The manufacturing process of biological medicines currently
One of the last remaining steps in the journey of a biological medicine to the patient is the registration process, i.e. the placing of the medicine on the market. The registration of biological medicines is currently assessed and approved by the European Medicines Agency.
The dispensing of all biological medicinal products is currently subject to prescription.
They are therefore not freely available in pharmacies, mainly due to their nature and safety in use.
Can we expect side effects with biological treatments?
As with all other medicines, there is a risk of side effects with biological treatments.
It should be remembered that side effects may not occur in every patient treated. The number and severity of side effects that do occur also varies.
The risk of occurrence and severity of side effects of biological medicines always depends on the type of treatment or the general health of the patient. The duration of side effects is usually short and they subside after a few hours or days.
A tabular summary of the most common adverse reactions to biological medicines
| Flu-like symptoms |
|
| Skin problems |
|
| Immune system disorders |
|
| Cardiovascular disorders |
|
| Side effects related to the injection route of administration |
|
| General disorders |
|
Each biologic drug has a different, specific pattern of possible side effects. Not all are included in the table above.
The occurrence of new, previously unreported adverse reactions is subject to ongoing monitoring by healthcare professionals and national regulatory authorities.
Some examples of biological medicinal products and their uses
As already mentioned, biologics have a wide range of uses in different areas of medicine.
To give you a better idea, here are some specific examples of substances and their use in the treatment of a particular health problem or disease.
A tabular overview of some biologics and their uses
| Biological drugs | Diseases |
| Interferons |
|
| Interleukin-2 |
|
| Tumour necrosis factor |
|
| Monoclonal antibodies | |
| Rituximab |
|
| alemtuzumab |
|
| ipilimumab |
|
| bevacizumab |
|
| cetuximab |
|
| trastuzumab | |
| etanercept | |
| infliximab |
|
| adalimumab |
|
| basiliximab |
|
| pexelizumab |
|
| erenumab, fremanezumab, galcanezumab | |
| omalizumab, mepolizumab, reslizumab, dupilumab | |
Interesting resources
Related