- pubchem.ncbi.nlm.nih.gov - Calcium
- ncbi.nlm.nih.gov - Calcium, Connie M. Weaver
- bones.nih.gov - Calcium and Vitamin D: Important at Every Age
- osteoporosis.foundation - Calcium
- ncbi.nlm.nih.gov - Calcium Intake and Health, Gabriela Cormick, Jose M Belizán
- ods.od.nih.gov - Calcium
- solen.cz - Calcium: bone and cardiovascular effects, MUDr. Mária Rašková, Ph.D.
- medicinapropraxi.cz - Calcium and vitamin D in primary and secondary prevention of osteoporosis, Pavel Novosad, M.D.
- adla.sk - CALCIUM (Ca)
What are the effects of calcium? Symptoms of deficiency, excess + food sources

Strong bones, healthy teeth. What is the importance of calcium in our lives? Why is it necessary and can it also be harmful?
Article content
- What is calcium?
- How is calcium produced?
- Do you know the function of calcium in the body?
- Calcium - from intake to excretion
- Why is vitamin D also important?
- Regulation of calcium levels in the body
- What levels of calcium are considered physiological?
- What should be the daily calcium intake?
- Sources of calcium - it doesn't have to be food
- When to supplement calcium with medications and supplements?
- Do you know the complications of high calcium intake?
What is calcium?
Calcium is a chemical element widely present in many components of our environment, including the human body.
It is the fifth most abundant element in the Earth's crust (making up over 3% of its content) and the third most abundant metal after iron and aluminium.
Calcium metal was first isolated by the English chemist Sir Humphry Davy in 1808 by electrolysis from a mixture of lime (CaO) and mercuric oxide (HgO).
Calcium is known by the chemical name Ca, which is derived from the Latin name calcium.
The name calcium comes from the Latin word calx. It translates as lime or limestone, in which calcium was discovered.
Calcium alloys have been known and used for a very long time (since BC), although people did not know their exact composition. They were mainly used in construction as a building material or as a sealing and bonding material.
Calcium is one of the alkaline earth metals. This group of elements is named after the fact that their oxides react with water to form alkalis, i.e. bases.
The alkaline earth metals include the elements of Group II A of the periodic table of the chemical elements (vertical columns). Calcium is classified in Period IV (horizontal rows).
In addition to calcium, strontium, barium and radium are also in this group of elements. All have very similar properties.
Although elements such as beryllium and magnesium are in the same group of the periodic table as calcium, they are not classified as alkaline earth metals. This is because they have different chemical and physical properties than the other elements in this group.
Calcium is a non-noble metal of silver colour, malleable and relatively soft. It can be cut with a knife with little effort. It has a low density and is capable of conducting an electric current. It burns with a characteristic yellow-red flame.
It is very reactive, forms compounds quite readily and therefore does not occur in nature in free form. It reacts spontaneously with water and reacts with oxygen and nitrogen on contact with air.
A tabular summary of basic chemical and physical information about calcium
| Name | Calcium |
| Latin name | Calcium |
| Chemical name | Ca |
| Classification of elements | Alkaline earth metal |
| Grouping | Solid |
| Proton number | 20 |
| Atomic mass | 40,078 |
| Oxidation number | +2 |
| Density | 1.54 g/cm3 |
| Melting point | 842 °C |
| Boiling point | 1484 °C |
| Hardness | 1,75 |

The most abundant calcium compound in nature is calcium carbonate (CaCO3). In this form, it is found in rocks such as limestone, dolomite, marble and chalk.
Other calcium compounds include:
- CaO - calcium oxide or quicklime
- Ca(OH)2 - calcium hydroxide or slaked lime
- CaSO4nH2O - calcium sulphate hydrates or gypsum
- CaSO4 - calcium sulphate or anhydrite mineral
- CaF2 - calcium fluoride or the mineral fluorite
- [Ca5(PO4)3F] - mineral
How is calcium produced?
Calcium and its compounds find use in many areas, from purely industrial uses to their function in the kitchen or cosmetics.
The largest producers of calcium are currently countries such as China, Russia, the USA, but also Canada and France.
In Russia and China, the Davy method of electrolysis is still used, with a slight variation of the starting compound (molten calcium chloride is used). In Western countries, a different production method is used.
It consists in displacing calcium atoms from calcium oxide (lime) by aluminium atoms in low-pressure vessels at high temperatures.
Do you know the function of calcium in the body?
Calcium is an essential part of the human body and an irreplaceable mineral for its proper functioning.
It makes up approximately 2% of the total body weight of the average adult.
Almost all of the calcium in the body (99%) is found in the bones and teeth. The remaining small amount is concentrated in the tissues and blood.
Calcium stored in the bones serves as a reservoir. When needed, it is used as a source of calcium for the body to maintain calcium balance.
The size of these stores is directly affected by the calcium balance in the body. It varies depending on calcium intake and absorption on the one hand and calcium loss through the skin, kidneys and intestines on the other.
In the bones and teeth, calcium is found in the form of the calcium ion hydroxyapatite. This mineral is the main inorganic component of hard dental tissues - tooth enamel, dentin and dental cement.
Calcium circulating in the blood is 33-50% bound to proteins, 5-15% is part of complexes and 50% of calcium in the blood is in ionised, i.e. free form (Ca2+).
At birth, 26-30 g of calcium is present in the body of a newborn. This amount increases rapidly thereafter. In adulthood, the calcium content in women is around 1200 g and in men around 1400 g.
In men, calcium levels remain constant throughout life. In women, they decrease due to menopause and the associated decrease in oestrogen production.
Since we already know where calcium is found in the body, we can deduce its physiological function.
So what is the main role of calcium in the human body?
Its main role is to participate in the construction of bones and teeth. It plays an important role in the growth, remodeling or repair of bone damage. It maintains the strength, power and flexibility of tissues and allows the body to perform natural movement.
The small amounts of calcium present in the bloodstream, muscles, extracellular fluid (fluid outside the cells) and in various tissues perform several other important functions.
- They affect the contraction and dilation of blood vessels.
- They regulate the activity of muscles and the heart.
- They are involved in blood clotting and in the transmission of nerve impulses.
- They regulate the production and secretion of hormones and enzymes.

Calcium - from intake to excretion
It is important to note that the human body is not capable of producing calcium on its own.
We are therefore dependent on its intake, whether in the form of daily food intake or its replacement in the form of drugs and supplements.
Calcium thus taken up is absorbed in the intestinal tract by two routes - active transport and passive diffusion.
Active transport through the intestinal cells requires energy and also the presence of vitamin D. It is particularly useful in low calcium intakes, i.e. in amounts up to 500 mg.
Passive diffusion does not consume energy. It is the dominant route of absorption at elevated calcium intakes, i.e. above 500 mg.
The rate of intestinal absorption of calcium in humans is generally low, approximately 45 % for intakes of 200 mg calcium or less per day and only approximately 15 % for intakes of more than 2000 mg calcium per day.
The rate of absorption is age-dependent. It is higher in childhood (approximately 60 %) because the significant growth and development of bones in children requires more calcium.
In adulthood, it drops to only 25 % and absorption decreases further with increasing age.
An acidic environment is necessary for calcium absorption. Its absorption is significantly reduced by dietary fibre, chelates and phytates.
Calcium losses in the blood are passive and are estimated to be approximately 100-150 mg/day.
The absorbed calcium is partly stored in the bones and partly excreted by the kidneys. Approximately 100 mg of calcium is excreted daily.
In connection with calcium and its fate in the body, it is also very important to mention vitamin D.
Why is vitamin D also important?
Vitamin D is classified as a steroid hormone based on its structure.
It plays a very important role in calcium metabolism and has a positive effect on the formation of healthy teeth and bones.
Vitamin D is primarily responsible for the absorption of calcium and phosphate from the intestines. It also regulates calcium utilization by increasing its absorption into the bones, which has a positive effect on their structure and mineralization.
This means that if we want to achieve sufficient levels of calcium in the body, it is necessary to think about sufficient intake and content of vitamin D.
Vitamin D deficiency leads to reduced absorption of calcium from the digestive tract, increased bone resorption (breakdown) and subsequent bone loss.
The main source of vitamin D is sunlight (90%), followed by dietary intake.
The skin absorbs the so-called provitamin D (precursor vitamin) by the action of sunlight. This is converted within a few hours into vitamin D3 - cholecalciferol. This is transported by the blood to the liver and converted by liver enzymes into the still inactive calcidiol. Only in the kidneys is it finally converted into the active molecule calcitriol.
The ability of the human body to synthesize vitamin D depends on the season, time in the sun, skin pigmentation, age, lifestyle, etc.
Dietary intake of vitamin D represents only about 5-10% of total intake. It is mainly found in marine fish, fish oil, meat, egg yolk, baked goods, fats and dairy products.
Regulation of calcium levels in the body
Almost all of the calcium in the body (99%) is stored in the bones, with a smaller amount in the blood, muscles and other tissues.
For proper functioning of daily life functions, the human body tries to maintain a constant level of calcium in the blood and tissues.
Three hormones - vitamin D, parathyroid hormone and calcitonin - are involved in maintaining physiological levels of calcium in the blood serum. These regulate the amount of calcium by acting on the intestines, kidneys and bones.
If the amount of calcium in the blood drops too low, the body sends a signal to the bones via parathyroid hormone.
Calcium is released from the bones into the blood, bringing the reduced calcium levels back to normal.
In addition to activating the bones, parathyroid hormone also triggers two other processes.
It activates vitamin D to increase the absorption of calcium from the digestive tract. At the same time, it reduces the excretion of calcium in the urine by acting on the kidneys.
Otherwise, if calcium levels in the body are high, another hormone - calcitonin (produced in the thyroid gland) - takes over.
This works in exactly the opposite way.
It lowers the level of calcium in the blood by stopping the release of calcium from the bones. It sends a signal to the kidneys to excrete more calcium in the urine.
What levels of calcium are considered physiological?
The amount of calcium present in the blood can be determined from blood serum or blood plasma. In healthy people, serum calcium levels should be approximately between 2.2 and 2.6mmol/l (8.8 and 10.4 mg/dl).
Physiological levels of calcium in ionised form range from 1.15 to 1.33 mmol/l (4.6 to 5.3 mg/dl).
Calcium can also be determined in urine.
Tabular summary of indicative reference values for blood and urine calcium determination
| Indicative reference values for calcium in serum or plasma | |
| Age < 10 days | 1.89-2.59 mmol/l |
| Age 10 days - 2 years | 2.24-2.74 mmol/l |
| Age 3-12 years | 2.19-2.69 mmol/l |
| Age 13-19 years | 2.30-2.75 mmol/l |
| Age 20-50 years | 2.20-2.55 mmol/l |
| Age over 50 years | 2.15-2.51 mmol/l |
| Indicative reference values for ionised calcium in serum or plasma | |
| Children and adults | 1,15-1,33 mmol/l |
| Indicative reference values for urinary calcium | |
| Children | < 0.15 mmol/kg in 24 hours |
| Adults | 2.5-8.0 mmol per 24 hours |
| 1.7-5.3 mmol/l (one urine dose) | |
Assessment of calcium in the body is also done by dual X-ray absorptiometry (DEXA for short). In this case, the level of calcium present in the bones is assessed and thus the nutritional status of the individual.
Any deviation from the physiological level of calcium in the body is considered a pathological condition.
1. Insufficient calcium levels in the body
A fall in serum calcium below the reference value of 2.12 mmol/l or a fall in ionised calcium below 1.15 mmol/l leads to hypocalcaemia.
Hypocalcaemia = reduced blood calcium level.
A tabular summary of the most common causes and symptoms of hypocalcemia
| Causes of hypocalcemia | Symptoms of hypocalcemia |
|
|
The most serious consequence of insufficient calcium levels in the body is the development of osteopenia, which is the first stage of the disease characterized by bone loss. If osteopenia is left untreated, osteoporosis develops.
In osteoporosis, there is a significant loss of bone mass and changes in the microarchitecture of the bones. This condition leads to a weakening of the strength of the bones and thus to increased brittleness.
Other consequences of low calcium levels include osteomalacia in adults and rickets in children. These are diseases leading to softening and deformation of the bones.
2. Excessive calcium levels in the body
The opposite of hypocalcaemia is a condition where the calcium level is above its reference value, i.e. above 2.75mmol/l (if the level is above 3.5mmol/l, it is already a serious condition). In this case, we speak of hypercalcaemia.
Hypercalcaemia = elevated blood calcium level.
A tabular overview of the most common causes and symptoms of hypercalcaemia
| Causes of hypercalcaemia | Symptoms of hypercalcaemia |
|
|
The most common consequences of hypercalcemia are kidney stone formation, kidney damage or failure, nerve disease and heart rhythm disturbances.
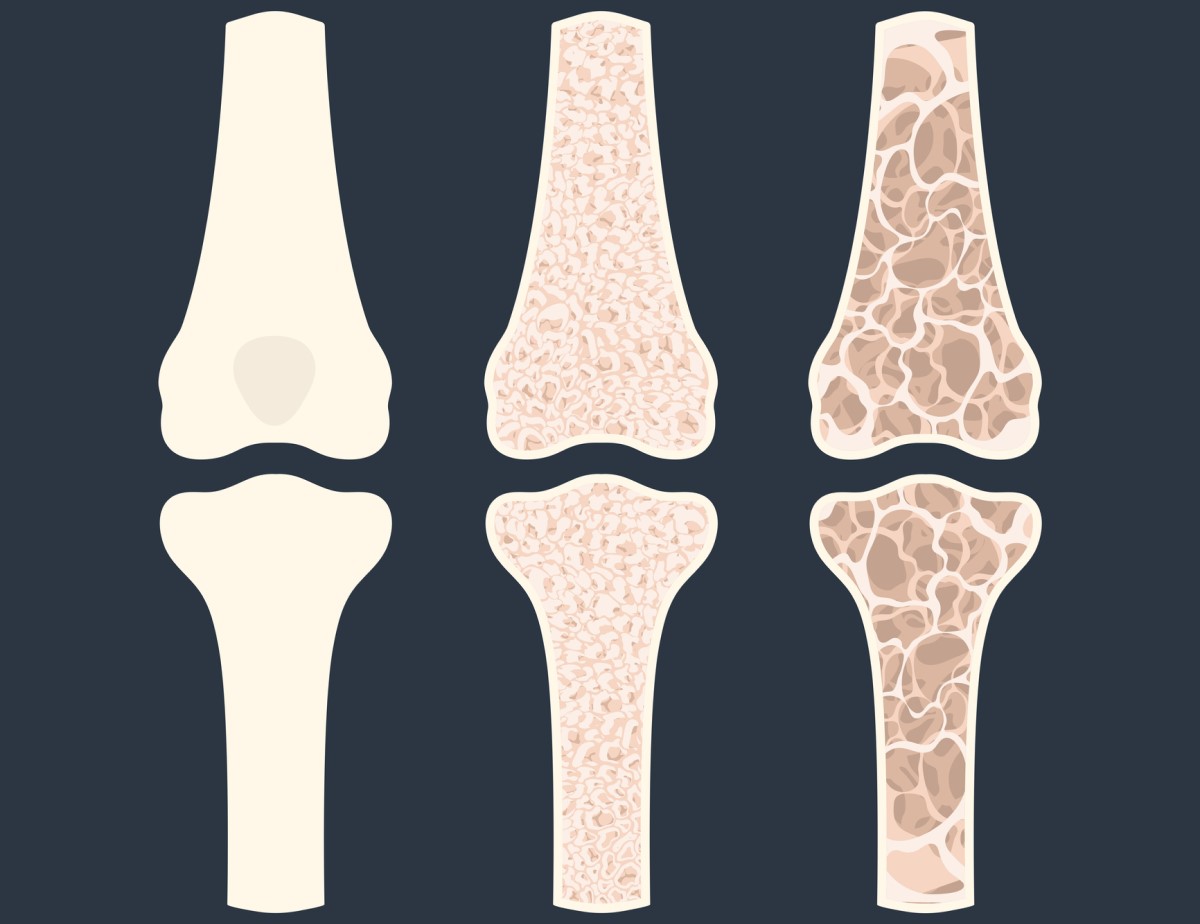
We now know the importance of calcium for the body and its optimal level. Let's take a closer look at how we can maintain or supplement the required level, or the recommended calcium intake, throughout the day.
What should be the daily calcium intake?
Calcium requirements vary considerably from one stage of life to another and depend mainly on age.
For example, the body's needs are higher in children or in adolescence. At this stage of life, significant growth and bone development occurs.
The need for calcium is also more pronounced in older people, as their ability to absorb calcium decreases due to the natural ageing process.
The human body obtains the calcium needed for optimal functioning in two ways.
The first way is by consuming calcium in a diet rich in this mineral or by supplementing it through medications and dietary supplements.
The second way is when the body has no other option and taps into its own calcium stores.
This occurs when adequate nutrition is not provided and the individual does not take in enough calcium-rich food.
If the body 'uses up' calcium from the bones due to inadequate food intake, this should be restored later when nutritional conditions improve. However, this method does not work so easily. It is not always possible to get calcium back into the bones simply by increasing calcium intake.
So how much calcium does a person need to take in daily in food or supplements to avoid the second scenario?
Let's look at this.
Table of recommended amount of calcium per day depending on age
| Age limit |
Amount of calcium recommended by FAO/WHO* |
Amount of calcium recommended by EFSA** |
| Children and adolescents | ||
| Age 0-6 months | 300-400 mg/day | Not applicable |
| Age 6-12 months | 300-400 mg/day | 280 mg/day |
| Age 1-3 years | 500 mg/day | 450 mg/day |
| Age 4-6 years | 600 mg/day | 800 mg/day |
| Age 7-10 years | 1 300 mg/day | 800 mg/day |
| Women | ||
| Age 11-14 years | 1 300 mg/day | 1 150 mg/day |
| Age 15-18 years | 1 300 mg/day | 1 150 mg/day |
| Age 19-24 years | 1 000 mg/day | 1 000 mg/day |
| Age 25-50 years | 1 000 mg/day | 950 mg/day |
| Age 51 years and over | 1 000 mg/day | 950 mg/day |
| During pregnancy and breastfeeding | ||
| Age 14-18 years | Not applicable | Same as for non-pregnant women |
| Age 19 years and over | 1 200 mg/day | Same as for non-pregnant women |
| Breastfeeding | 1 000 mg/day | Same as for non-lactating women |
| Men | ||
| Age 11-14 years | 1 300 mg/day | 1 150 mg/day |
| Age 15-18 years | 1 300 mg/day | 1 150 mg/day |
| Age 19-24 years | 1 000 mg/day | 1 000 mg/day |
| Age 25-50 years | 1 000 mg/day | 950 mg/day |
| Age 51 years and over | 1 000 - 1300 mg/day | 950 mg/day |
| ||
Maximum daily calcium intake should not exceed 2 500-3 000 mg/day for adolescents, 2 500 mg/day for adults and 1550 mg/day for children.
It is very important that an individual's daily calcium intake is as close as possible to the recommended amount.
This is particularly relevant for the following age groups:
- Infants - A critical element in determining the recommended daily calcium intake is whether the infant is breastfed or on breastmilk substitutes. Calcium intake should be slightly higher in infants receiving breastmilk substitutes. This is because of the lower efficiency of calcium absorption from breastmilk substitutes.
- Younger children and pubertal children - Have a higher calcium requirement due to development and growth.
- Menopausal women - Increased calcium requirements are due to a decrease in estrogen levels and therefore an increased risk of osteoporosis.
- Older people - Have a higher loss of calcium from bones, which is a natural part of aging.
Sources of calcium - it doesn't have to be food
The most common source of calcium for humans is food. A healthy person can get enough calcium in a daily balanced diet that is rich in calcium.
The main calcium-rich foods are dairy products (cow, sheep, goat) - milk, yoghurt, cheese, cottage cheese, etc. These can provide up to 40% of the total daily calcium intake in a single serving.
Which other foods are considered to be rich in calcium and are therefore suitable for achieving the recommended daily calcium intake?
- Green vegetables - broccoli, kale, Chinese cabbage, spinach, mustard
- Nuts and seeds - almonds, sesame seeds
- Canned fish with bones - sardines, salmon
- Eggs
- Legumes - beans, lentils
- Fruit and fruit juices
- Bread, cereals
- Mineral waters
- Soya and soya drinks
- Tofu
- Calcium fortified products
Calcium content varies from food to food.

It is also important to note that many other foods or their contents can interfere with the process of calcium absorption or excretion in the body.
Certain plant-derived substances, such as stellate acid and phytic acid, significantly reduce calcium absorption in the digestive tract. These bind with calcium to form indigestible salts. Persons whose diet is predominantly plant-based should therefore be particularly vigilant.
Calcium absorption is also reduced to a lesser extent in the presence of caffeine and phosphorus.
High amounts of protein and sodium (salt) may increase calcium excretion by the kidneys.
People who are lactose intolerant are at risk of inadequate dietary calcium intake.
These people are unable to process lactose because of a lack of the enzyme lactase, which breaks lactose down into simpler sugars. Therefore, they avoid consuming dairy products.
In addition to food, calcium can also be taken in through medications or supplements containing calcium.
When to supplement calcium with medications and supplements?
Although a balanced diet is certainly a better and preferred way of getting calcium, in some cases it is necessary to get calcium into the body in a different way - through medications or calcium-containing supplements.
These products are designed for people who:
- Do not get enough calcium from food, for example because of a food allergy, intolerance or diet
- Have digestive or stomach disorders in which the ability to absorb calcium may be impaired
- Have an increased risk of calcium deficiency
- Have osteoporosis or an increased risk of osteoporosis
- Have thyroid disease
- Have chronic renal failure
In addition, the use of calcium preparations is recommended for people on long-term corticosteroid treatment.
The amount of calcium that needs to be replaced in the form of medications and supplements should be based on how much calcium a person is able to take in from food.
There is currently a wide range of calcium-containing products on the market, either in single-ingredient form or as part of multivitamin preparations. They can also be found in various dosage forms - tablets, capsules, powder, syrup, suspension, injectable forms, etc.
For medical purposes, calcium is most commonly used in the form of calcium acetate, calcium carbonate, calcium chloride, calcium citrate, calcium glubionate, calcium gluconate or calcium lactate. These differ in the presence of elemental calcium.
For example, calcium carbonate contains 40 % elemental calcium
calcium citrate 21 %
calcium lactate 13 %
and calcium gluconate 9 %.
A number of useful guidelines should be followed when using products containing calcium.
- Take them together with vitamin D.
- Take smaller doses (approximately 500 mg) several times a day. Taking smaller doses allows for better absorption of calcium in the digestive tract.
- Take them at the same time as food, as absorption and tolerance are improved (in the case of calcium citrate, sufficient calcium absorption is maintained even when taken without food).
- An acidic environment is necessary for better absorption of calcium. This should be taken into account especially in persons who have a problem with reduced or insufficient acid production in the stomach.
- Avoid the simultaneous use of preparations containing calcium and iron.
Consult your doctor about the need for and choice of calcium preparations.
Your doctor will also help you choose the most appropriate and safest product, taking into account your medical condition and the medications you are currently taking (e.g., blood pressure, thyroid, bone disease, antibiotics, etc.).
In addition to drugs whose primary indication is to replace a calcium deficiency in the body, there are also drugs on the market containing calcium for other uses. We are talking about antacids - drugs designed to neutralise, i.e. reduce the acidity of gastric juices.
Do you know the complications of high calcium intake?
Prolonged and excessive intake of high amounts of calcium leads to health complications.
These are mainly manifested in the initial stages by digestive problems such as constipation, bloating and flatulence, abdominal pain or diarrhoea.
Skin problems in the form of rashes, itchy skin or hives may also occur.
Sometimes excessive calcium intake is also manifested by an increase in the level of calcium in the urine. This is called hypercalciuria. In rare cases, hypercalcaemia may develop.
Interesting resources
Related