- pubchem.ncbi.nlm.nih.gov - Chlorine
- ocw.mit.edu - CHLORINE & ITS CONSEQUENCES, Jacqueline Brazin
- pubmed.ncbi.nlm.nih.gov - A Quick Reference on Chloride, Andrea A Bohn, Helio Autran de Morais
- pubmed.ncbi.nlm.nih.gov - Hyperchloremia - Why and how, Glenn T Nagami
- sciencedirect.com - Water and electrolyte balance, Anna E Merrill, Allison B Chambliss
- multimedia.efsa.europa.eu - Dietary Reference Values for the EU
- medlineplus.gov - Chloride - Urine test
- solen.sk - Chlorine (Cl-) and ammonia (NH3) intoxication, MUDr. Viliam Dobiáš, PhD
- solen.sk - Acid-base balance (acid-base), MUDr. Ján Kozánek
What role does chlorine play in our bodies? Is it safe for our bodies?

Photo source: Getty images
Do you know the effects of chlorine on our bodies? Is this chemical element beneficial or even necessary for our health? Which chlorine compounds are essential for us and which ones pose a health risk?
Article content
- Basic properties of chlorine
- Can chlorine pose a health risk?
- How do chlorides affect our health?
- How does the human body treat chloride?
- Do you know the source of chloride in food?
- What is the recommended daily intake of chloride?
- What causes deficiency and excess chloride in the body?
- Insufficient chloride in the blood
- Excessive amounts of chloride in the blood
- Abnormal levels of chloride in the urine
Basic properties of chlorine
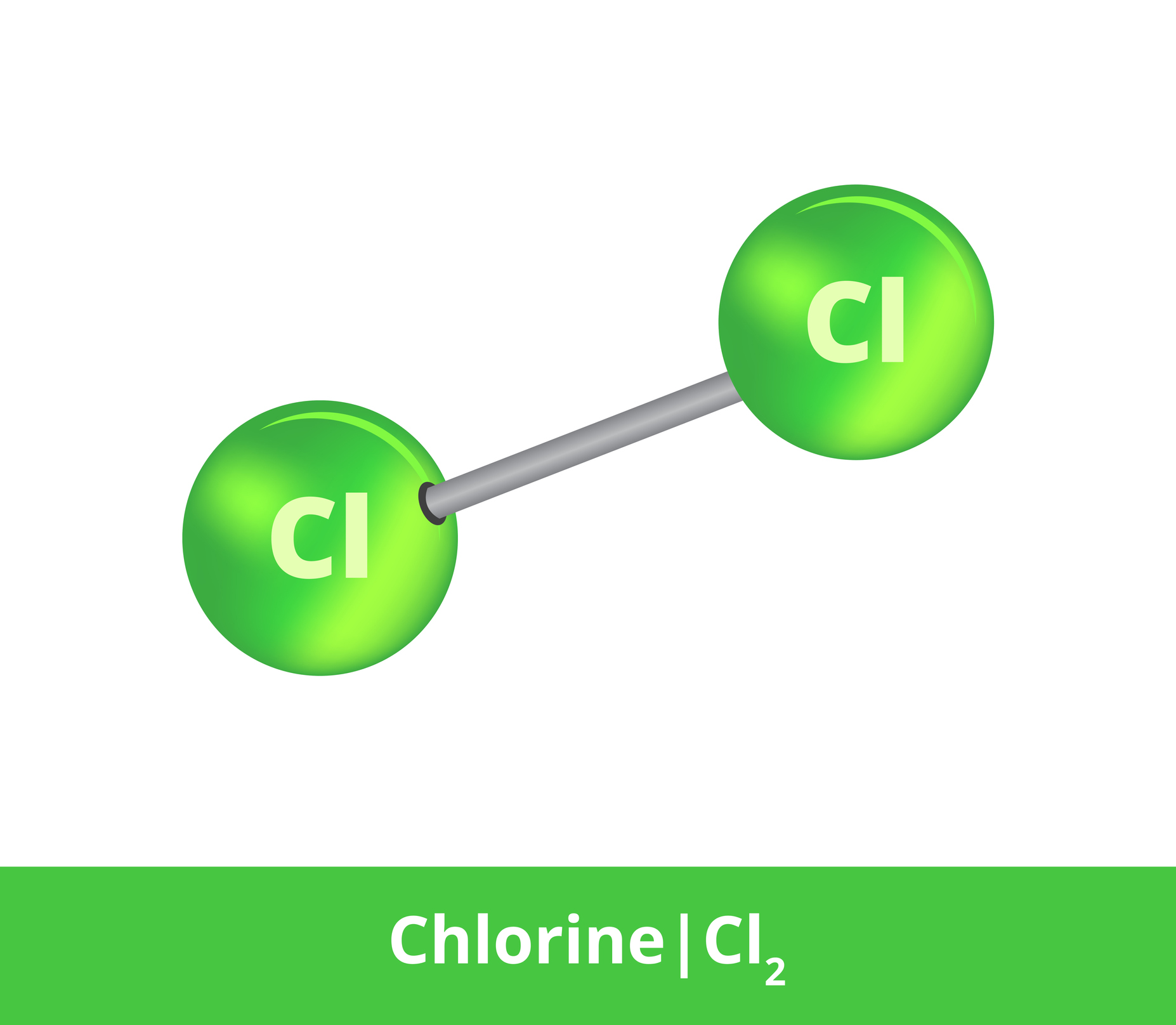
Chlorine is a chemical element known by the chemical symbol Cl. It is derived from the Latin word chlorum. It comes from the Greek word khloros, which translates as greenish yellow.
The name refers to its appearance. It's a greenish-yellow gas. It has a very unpleasant irritating and suffocating smell.
Chlorine is an element of group 17 of the periodic table of chemical elements and is found in period 3.
It belongs to a group of elements called halogens, which also includes fluorine, bromine, and iodine. The group was named for the ability of its elements to form salts (from the Greek hals - sol, gennaó - I form).
In terms of abundance, chlorine is the second most abundant halogen (after fluorine) and the 21st most abundant chemical element in the Earth's crust.
It was discovered in 1774 by the Swedish chemist Carl Wilhelm Scheele, who reacted manganese dioxide with hydrochloric acid. However, Carl Wilhelm Scheele mistakenly believed that it was not pure chlorine, but a compound of chlorine with oxygen.
The fact that the reaction produced a pure chemical element was not proven until 1810 by the English chemist Sir Humphry Davy, who also gave it the name chlorine.
Chlorine is a very reactive element. It is soluble in water, heavier than air and very poisonous. At room temperature and atmospheric pressure it is not explosive. At low temperatures or under pressure it turns into a liquid.
It has strong oxidizing, bleaching and disinfecting properties.
It is therefore currently used as a disinfectant, e.g. in disinfectants, to treat drinking water or swimming pool water, also as an insecticide.
Chlorine is also widely used in the paper, paint and textile industries.
It is used in the production of the versatile plastic PVC (polyvinyl chloride), in organic chemistry as an oxidising agent or in the pharmaceutical industry in the manufacture of pharmaceuticals.
In the past, chlorine compounds were used, for example, to create paintings known as the first photographs or in medicine as an anaesthetic (chloroform).
During World War I, the highly poisonous effects of chlorine gas were exploited and it was used as a chemical weapon (yperite).
A tabular summary of basic chemical and physical information about chlorine
| Name | Chlorine |
| Latin name | Chlorine |
| Chemical name | Cl |
| Classification of elements | Halogen |
| Grouping | Gas (at room temperature) |
| Proton number | 17 |
| Atomic mass | 35,45 |
| Oxidation number | -1, +1, +3, +5, +7 |
| Density | 3,2 g/l |
| Melting point | -101,5 °C (as Cl2) |
| Boiling point | -34,04 °C (as Cl2) |
Due to its reactivity, chlorine does not occur in monoatomic form, but always in bound form or as a diatomic Cl2 molecule.
The most common chlorine compound is sodium chloride, known as table salt.
In nature, chlorine occurs mainly in seawater. It also occurs as a component of minerals such as halite (NaCl), sylvinite (KCl), bischofite (MgCl2-6H2O), carnallite (KCl-MgCl2-6H2O) or kainite (KCl-MgSO4-3H2O).
Can chlorine pose a health risk?
On the one hand, chlorine as an element is essential for the human body for the proper functioning of many physiological processes. On the other hand, it poses a dangerous health risk.
Excessive exposure to the diatomic chlorine molecule Cl2 causes intoxication of the body. Chlorine enters the body by inhaling contaminated air, eating contaminated food or through skin contact.
The extent of chlorine exposure depends on the amount of chlorine and the duration and frequency of exposure. Chlorine is irritating to the respiratory tract, eyes and skin.
The first symptoms of intoxication are coughing, shortness of breath, choking, headache, nausea, dizziness, burning eyes, watery eyes, abdominal pain and muscle weakness.
In more severe poisoning, conjunctivitis, inflammation of the conjunctivae, nose, pharynx, larynx and bronchi, corneal ulceration, increased heart rate, swelling of mucous membranes and lungs leading to oxygen deprivation occur.
In milder forms of intoxication, irritation of the eyes and respiratory tract occurs. Moderate poisoning is characterised by systemic symptoms. Severe poisoning may be life-threatening or have long-term or permanent consequences.
On the other hand, when is chlorine beneficial or necessary for our health?
Chlorine is present in the human body in the form of its salts. In these we find chlorine in the form of the negatively charged chloride anion Cl-.
Salts containing chloride anion are called chlorides.
How do chlorides affect our health?
Chlorides are an essential component of the human body. They play an important role in digestion, muscle and nerve function, regulation of body fluids, and in maintaining acid-base balance.
Total body fluid makes up approximately 60% of a person's weight and consists of three components - intracellular fluid (the fluid inside cells), blood plasma and tissue fluid.
Blood plasma and tissue fluid together form extracellular fluid, i.e. fluid outside the cells.
The intracellular and extracellular fluids are separated by cell membranes. They differ in their water, electrolyte and protein content. However, these differences are only minimal.
Electrolytes present in body fluid include positively charged ions such as sodium, potassium, calcium, or magnesium, and negatively charged ions such as chloride, phosphate, or bicarbonate.
Water moves freely across cell membranes, electrolytes through transport systems.
Maintaining a balance in the volume and composition of body fluids, i.e., intracellular and extracellular, is crucial to the existence of cells and their normal function.
Disturbance of this balance causes an increase or decrease in the concentration of electrolytes inside or outside the cells and consequently the development of certain disorders.
Chloride represents the most important and abundant anion in the extracellular fluid. It contributes significantly to the maintenance of the overall fluid balance in the body.
Up to 70% of all negatively charged ions in the body are chloride.
In addition, chloride also contributes to the maintenance of acid-base balance in the body, i.e. the ratio of acidic to basic components.
The acidity of the internal environment is strictly regulated. The physiological pH of blood and other body fluids is just around 7.4.
Changes from this pH value affect the properties and activity of proteins, enzymes, transporters or membrane channels.
If acidic components predominate, we speak of a state of acidosis; conversely, if alkaline components predominate, we speak of a state of alkalosis.
A physiological level of chloride helps to maintain the pH at normal values. Any significant decrease or increase in chloride levels results in the development of disturbances in the acid-base balance.
What are the other indispensable functions of chloride for humans?
- Chloride ions are essential for the production of stomach acid - hydrochloric acid (HCl), which is involved in the digestion of food, and also acts as a preventative against bacterial overgrowth in the digestive tract.
- In the form of gastric acid, they are involved in the activation of the enzyme pepsinogen in the gastric environment, which is necessary for the absorption of important substances from food.
- They contribute to the maintenance of proper osmotic pressure.
- They help the contraction of muscles, including the heart muscle.
- They are essential in the transmission of signals in the nervous system.
- They are involved in the exchange of oxygen and carbon dioxide in red blood cells.
- They also affect potassium levels in the body.
How does the human body treat chloride?
Chloride anions are absorbed into the body from food in the digestive tract. Almost all chloride in food is absorbed into the blood.
Chloride concentration is not controlled by homeostasis, unlike sodium or potassium, for example.
The amount of chloride present in the body depends on the presence of other important ions. The concentration of chloride varies in parallel with the actual amount of these ions. These are mainly sodium ions.
This close relationship between chloride and sodium levels is mainly due to the fact that the main source of both ions is sodium chloride (table salt).
Chloride is mainly found in the extracellular fluid (it makes up to two-thirds of the anions present in the blood). It is deposited to a small extent in the skin, subcutaneous tissues and skeleton.
Reference values for chloride in blood serum or plasma range from 98 to 111 mmol/l.
In terms of regulating their levels, the kidneys are the main organ responsible for maintaining chloride balance.
The amount of chloride excreted in the urine depends on the degree of filtration in the kidneys and also on a number of transport processes taking place in the kidney cells.
Chloride excretion is also closely related to sodium excretion.
The rate of urinary chloride excretion also corresponds to the daily dietary intake of chloride, including fluid intake. It ranges from approximately 110 to 250 mmol/day.
In addition to the loss of chloride in the urine, other routes of excretion from the body are known. This is via faeces or sweat.
Although losses by sweat are negligible under normal conditions, they must be taken into account in case of increased sweating in high temperatures or excessive physical activity.
Do you know the source of chloride in food?
Chloride is mostly ingested through food. Food can sufficiently cover a person's daily requirement.
The main source is sodium chloride, known as table salt. It is used daily in the production and processing of food and in the preparation and flavouring of food.
Inadequate dietary intake of chloride is relatively rare. Conversely, the current trend is towards high to excessive salt intake, which can ultimately lead to high blood pressure, heart disease and kidney disease.

The total amount of chloride intake in the diet is a combination of:
- the small amount of chloride that is naturally present in the diet
- larger amounts of chloride that are used in the form of salt in food preparation or for flavouring
- an even larger amount of chloride added to food during its production and processing in the form of salt
Among foods, fruits, vegetables (tomatoes, olives, lettuce, celery), cereals (rye), milk, eggs, seaweed, unprocessed fish and meat are relatively rich sources of chloride.
It should be borne in mind that food processing, which usually involves the addition of salt, increases the proportion of chloride contained.
What is the recommended daily intake of chloride?
According to the European Food Safety Authority, the recommendations for a safe and adequate daily intake of chloride are as follows.
Table of daily chloride intake by age
| Age group | Intake of chloride |
| Infants (aged 7-11 months) | Not specified |
| Children (aged 1-3 years) | 1.7 g/day |
| Children (aged 4-6 years) | 2 g/day |
| Children (aged 7-10 years) | 2.6 g/day |
| Adolescents (aged 11-17 years) | 3.1 g/day |
| Adults (age = 18 years) | 3.1 g/day |
| Pregnant women (age = 18 years) | 3.1 g/day |
| Breastfeeding women (age = 18 years) | 3.1 g/day |
What causes deficiency and excess chloride in the body?
Deviations from the above physiological levels of chloride in the blood or urine are considered a medical condition.
Two situations can occur - the presence of excessive amounts of chloride in the blood and urine or a deficiency.
Compared to other minerals essential to the body, such as sodium or potassium, deviations in blood chloride levels are not associated with serious health consequences.
Assessment of chloride levels is a useful indicator in the diagnosis of acid-base disturbances or in the determination of anion deficiency in extracellular fluid.
The concentration of chloride anions in the blood usually tracks the state of sodium cation concentration. That is, if sodium concentration decreases, chloride concentration decreases. Conversely, if sodium concentration increases, chloride concentration increases.
Insufficient chloride in the blood
A condition characterised by insufficient chloride in the blood is called hypochloremia. Hypochloremia is said to occur when the chloride in the blood serum or plasma falls significantly below the reference value of 98 mmol/l.
The drop is usually due to one of the following situations:
- When the body has an inadequate dietary intake of chloride.
- If chloride absorption is impaired.
- If there is excessive chloride excretion.
- If the body is unable to use sufficient chloride efficiently.
Chloride deficiency due to inadequate dietary chloride intake is very rare. This mineral is present in many foods and especially in frequently used table salt.
Reduced or inadequate absorption may be due to specific metabolic diseases or diseases of the gastrointestinal tract causing reduced absorption capacity.
Increased chloride excretion occurs when:
- Conditions associated with severe nausea and vomiting
- Excessive sweating
- Burns
- Kidney disease
- Use of certain medications, such as drugs that increase urine production and excretion or corticosteroids
- Conditions of severe alkalosis, where alkaline components predominate in the body
- Respiratory diseases that are characterized by the presence of alkalosis, such as asthma, pulmonary edema, chronic obstructive pulmonary disease. In these, there is reduced reabsorption of chloride in the kidneys back into the blood.
Hypochloremia can also be caused by diseases and disorders in which the body retains too much water or whose direct consequence is reduced sodium levels. In fact, chloride and sodium levels are closely linked.
The treatment of hypochloremia consists primarily of treating the cause, i.e. the disease or disorder. In some cases, the administration of chloride-containing solutions may be necessary.
Excessive amounts of chloride in the blood
The opposite of hypochloremia is a condition where there is an excessive amount of chloride in the blood. This means significantly more than the reference value of 111 mmol/l. This time we are talking about hyperchloremia.
Hyperchloremia can occur for a number of reasons. The most common include:
- Excessive administration of chloride, e.g. in the form of infusion of sodium chloride solution, potassium chloride, parenteral nutrition (nutrition given into a vein)
- Administration of hypertonic (highly concentrated) solutions
- Excessive loss of water from the body (dehydration). May occur with hot flashes, excessive sweating, inadequate fluid intake, drowsiness, excessive urination.
- Hyperchloremia associated with metabolic acidosis, where acidic components predominate in the body. It occurs e.g. in kidney disease, when acidic substances are administered into the blood, in some forms of diarrhoea.
- Kidney disease associated with chloride retention
- Excessive loss of sodium through the digestive tract
- Intoxication with iodine or bromine salts
Diseases and disorders that directly lead to elevated sodium levels can also be the cause of hyperchloremia.
The treatment of hyperchloremia also consists primarily in eliminating its cause. This may include, for example, the administration of alkaline salts, support for kidney function and others.
Abnormal levels of chloride in the urine
In addition to abnormal levels of chloride in the blood, whether we are talking about increased or decreased amounts, we can also encounter deviations from their physiological levels in the urine.
The level of chloride in the urine that is considered normal is in the range of 110-250 mmol per day.
These values can be adjusted to some extent. Chloride levels may fluctuate slightly outside this range, depending on the amount of salt and fluid a person takes in daily.
There may be several reasons for fluctuations in urinary chloride levels.
A tabular summary of the most common causes of decreased and increased urinary chloride levels
| Decreased urinary chloride levels | Increased urinary chloride levels |
|
|
Interesting resources
Related

PharmDr. Marianna Forgáčová
Doctor of Pharmacy
I graduated from the Faculty of Pharmacy of Comenius University in Bratislava with a 5-year degree in Pharmacy, with a degree of Mgr. After graduation I worked at the State Institute for Drug Control in the section of drug registration from 2016, where I stayed for 4 years. During my employment I completed my rigorous studies again at the Faculty of Pharmacy in Bratislava. This time with the degree of PharmDr. Since 2020, I have been employed at Essity Slovakia s.r.o., where I work as an expert in registration processes and legislation related to medical devices. I consider my studies and practical experience as a sufficient prerequisite for providing professional and relevant information on medical topics. In general, I am most interested in the topics of medicines, pharmaceuticals or medical devices, or in legislation and registration issues in the field of pharmacy.
View all articles by the same authorThe aim of the portal and content is not to replace professional
examination. The content is for informational and non-binding purposes
only, not advisory. In case of health problems, we recommend seeking
professional help, visiting or contacting a doctor or pharmacist.